Oxide compounds play a crucial role in various industrial and biological processes due to their unique chemical properties and widespread occurrence. Understanding oxide formation and behavior can enhance your knowledge in materials science, corrosion resistance, and environmental chemistry. Explore this article to discover how oxides impact technology and nature in everyday life.
Table of Comparison
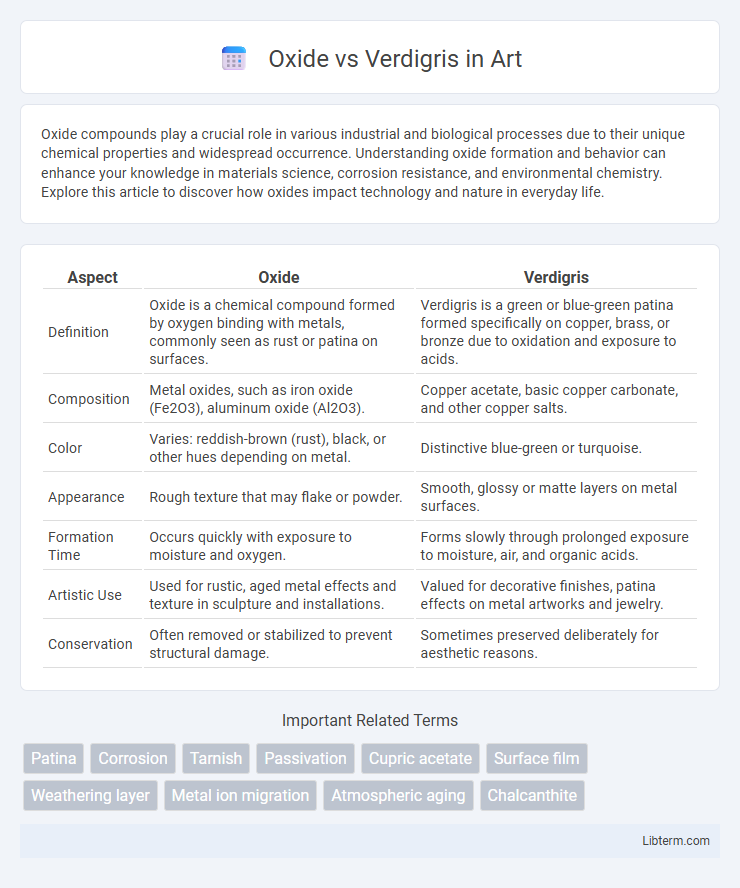
| Aspect | Oxide | Verdigris |
|---|---|---|
| Definition | Oxide is a chemical compound formed by oxygen binding with metals, commonly seen as rust or patina on surfaces. | Verdigris is a green or blue-green patina formed specifically on copper, brass, or bronze due to oxidation and exposure to acids. |
| Composition | Metal oxides, such as iron oxide (Fe2O3), aluminum oxide (Al2O3). | Copper acetate, basic copper carbonate, and other copper salts. |
| Color | Varies: reddish-brown (rust), black, or other hues depending on metal. | Distinctive blue-green or turquoise. |
| Appearance | Rough texture that may flake or powder. | Smooth, glossy or matte layers on metal surfaces. |
| Formation Time | Occurs quickly with exposure to moisture and oxygen. | Forms slowly through prolonged exposure to moisture, air, and organic acids. |
| Artistic Use | Used for rustic, aged metal effects and texture in sculpture and installations. | Valued for decorative finishes, patina effects on metal artworks and jewelry. |
| Conservation | Often removed or stabilized to prevent structural damage. | Sometimes preserved deliberately for aesthetic reasons. |
Introduction to Oxide and Verdigris
Oxide is a compound formed when a metal reacts with oxygen, commonly resulting in a protective or corrosive layer on the metal's surface. Verdigris, a specific type of greenish-blue patina, develops primarily on copper, brass, or bronze due to prolonged exposure to moisture and carbon dioxide. Both substances represent natural chemical changes but differ significantly in composition and visual appearance.
Understanding Oxide: Definition and Formation
Oxide is a chemical compound formed when a material such as metal reacts with oxygen, resulting in a stable molecule like iron oxide or aluminum oxide. This process, known as oxidation, involves the transfer of electrons and typically occurs in the presence of moisture or air, causing a surface layer that can either protect the metal or lead to corrosion. Understanding oxide formation is crucial in fields like metallurgy and materials science because it impacts metal durability, appearance, and performance.
What is Verdigris? Origins and Characteristics
Verdigris is a greenish-blue patina that forms on copper, brass, or bronze surfaces due to prolonged exposure to air, moisture, and acidic conditions. This natural oxidation process results in copper salts such as copper acetate or copper carbonate, giving verdigris its distinctive color and protective layer. Often found on historic sculptures and architectural elements, verdigris is valued for its aesthetic appeal and chemical stability.
Chemical Processes: Oxidation vs Verdigris Formation
Oxidation is a chemical process where metal atoms, typically copper or iron, react with oxygen to form a thin layer of oxide, such as copper oxide or iron oxide. Verdigris forms through further chemical reactions when copper oxide interacts with moisture, carbon dioxide, and atmospheric pollutants, resulting in a greenish patina primarily composed of copper carbonate compounds. This transformation from oxide to verdigris involves complex environmental factors accelerating metal corrosion and surface alteration.
Common Metals Prone to Oxide and Verdigris
Common metals prone to oxide formation include iron, aluminum, and copper, where oxidation results in rust, aluminum oxide, and a protective layer on copper, respectively. Verdigris specifically develops on copper, brass, and bronze due to prolonged exposure to moisture and acidic conditions, forming a distinctive green patina. Understanding the difference highlights that oxide refers to the broader category of metal oxides, while verdigris is a specific type of copper corrosion.
Visual Differences: Oxide vs Verdigris
Oxide typically appears as a uniform layer of black, brown, or reddish coating on metals like copper or iron, resulting from oxidation processes, whereas verdigris is characterized by its distinct bluish-green or turquoise patina formed specifically on copper, brass, or bronze due to prolonged exposure to moisture and atmospheric elements. The texture of oxide layers tends to be matte and can be flaky or powdery, while verdigris often exhibits a smoother, sometimes crusty surface with vibrant color variations ranging from green to blue-green. These visual differences are crucial for identifying metal corrosion stages and types, aiding in material conservation and restoration efforts.
Environmental Factors Influencing Formation
Oxide and verdigris formation are significantly influenced by environmental factors such as humidity, temperature, and exposure to pollutants. Oxide layers typically develop in dry, oxygen-rich environments, whereas verdigris forms in moist, acidic conditions where copper compounds react with carbonates or chlorides. Urban pollution, salt spray near coastal areas, and industrial emissions accelerate verdigris formation by providing chloride and sulfate ions that facilitate the patination of copper and bronze surfaces.
Impact on Metal Durability and Longevity
Oxide corrosion forms a protective layer on metals like aluminum and iron, often enhancing durability by preventing further oxidation. Verdigris, a green patina primarily on copper and brass, acts as a barrier but can be corrosive if moisture penetrates, potentially reducing metal longevity. Understanding the specific chemical composition and environmental exposure is crucial for assessing the impact of oxide versus verdigris on metal lifespan.
Removal and Prevention Techniques
Oxide buildup, primarily iron oxide, requires mechanical removal methods such as wire brushing or sanding, followed by applying rust converters or inhibitors to prevent recurrence. Verdigris, a green patina of copper carbonate, is best removed using mild acids like vinegar or lemon juice, while sealing with protective coatings or lacquer prevents further corrosion. Regular maintenance and environmental control, including reducing moisture exposure and using protective barriers, are essential for long-term prevention of both oxides and verdigris.
Conclusion: Choosing Between Oxide and Verdigris
Choosing between oxide and verdigris depends on the desired aesthetic and application context; oxide provides a more uniform, matte finish ideal for industrial or modern designs, while verdigris offers a distinctive greenish-blue patina that adds vintage or artistic character to metal surfaces. Consider durability and maintenance requirements, as oxide coatings tend to be more stable and protective against corrosion, whereas verdigris patinas may require periodic sealing to preserve their appearance. Selecting the right option involves balancing visual impact with functional longevity to suit the specific project needs.
Oxide Infographic
 libterm.com
libterm.com