Planktonic cells are free-floating microbial cells that exist independently in liquid environments, playing a crucial role in nutrient cycling and ecosystem dynamics. These cells exhibit different behaviors and susceptibilities compared to their biofilm-associated counterparts, impacting both natural processes and medical treatments. Explore the article to understand how planktonic cells influence your environment and health.
Table of Comparison
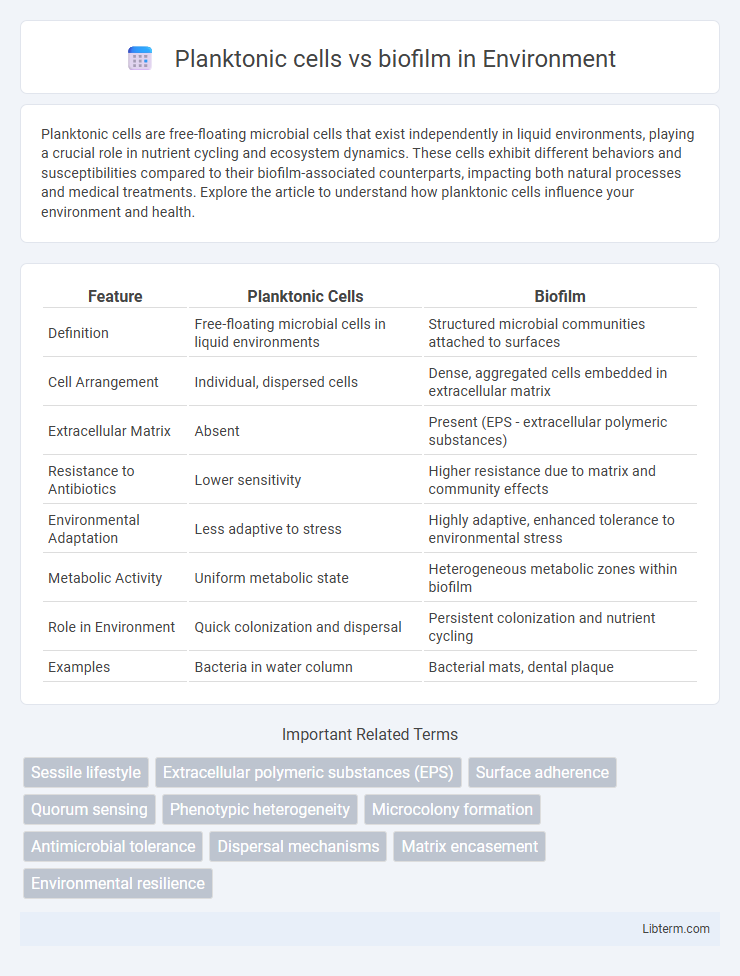
| Feature | Planktonic Cells | Biofilm |
|---|---|---|
| Definition | Free-floating microbial cells in liquid environments | Structured microbial communities attached to surfaces |
| Cell Arrangement | Individual, dispersed cells | Dense, aggregated cells embedded in extracellular matrix |
| Extracellular Matrix | Absent | Present (EPS - extracellular polymeric substances) |
| Resistance to Antibiotics | Lower sensitivity | Higher resistance due to matrix and community effects |
| Environmental Adaptation | Less adaptive to stress | Highly adaptive, enhanced tolerance to environmental stress |
| Metabolic Activity | Uniform metabolic state | Heterogeneous metabolic zones within biofilm |
| Role in Environment | Quick colonization and dispersal | Persistent colonization and nutrient cycling |
| Examples | Bacteria in water column | Bacterial mats, dental plaque |
Introduction to Planktonic Cells and Biofilms
Planktonic cells are free-floating microbial cells that exist independently in aqueous environments, allowing rapid dispersion and colonization. Biofilms are structured microbial communities embedded in a self-produced extracellular matrix, adhering to surfaces and exhibiting enhanced resistance to antibiotics and environmental stresses. Understanding the differences between planktonic cells and biofilms is crucial for developing effective strategies to control microbial infections and prevent biofilm-associated device contamination.
Defining Key Differences: Planktonic vs Biofilm States
Planktonic cells exist as free-floating, single bacterial cells thriving in liquid environments, whereas biofilms are structured communities of bacteria encased in a self-produced extracellular matrix attached to surfaces. Planktonic cells exhibit high metabolic activity and are more susceptible to antibiotics, contrasting with biofilms that demonstrate increased resistance due to their protective matrix and altered gene expression. The transition from planktonic to biofilm state involves complex signaling pathways like quorum sensing, enhancing bacterial survival under hostile conditions.
Structural Features of Planktonic Cells
Planktonic cells are free-floating microbial cells that exhibit a single, often spherical or rod-shaped morphology with a smooth and uniform cell surface, allowing rapid movement and nutrient uptake in liquid environments. Unlike biofilm-associated cells, planktonic cells lack the extracellular polymeric substance (EPS) matrix that provides structural support and protection in biofilms. These cells rely on flagella or pili for motility, facilitating dispersion and colonization but making them more susceptible to environmental stresses and antimicrobial agents.
Biofilm Architecture and Composition
Biofilm architecture features a complex, structured community of microbial cells embedded in a self-produced extracellular polymeric substance (EPS) matrix, primarily composed of polysaccharides, proteins, and extracellular DNA. This matrix provides mechanical stability, nutrient retention, and protection against environmental stresses, contrasting with the single-cell, planktonic lifestyle where cells exist freely suspended without such structural organization. The spatial heterogeneity and chemical gradients within biofilms create microenvironments that influence metabolic activity and gene expression, enhancing resilience and persistence compared to planktonic cells.
Environmental Adaptations and Survival Strategies
Planktonic cells exhibit rapid mobility and disperse widely to exploit transient nutrient sources, relying on quick reproduction and metabolic flexibility to survive fluctuating environments. Biofilm communities enhance protection against environmental stressors through extracellular polymeric substance matrices that shield against desiccation, antibiotics, and immune responses, promoting long-term stability. This sessile lifestyle within biofilms allows cells to engage in cooperative interactions, genetic exchange, and resource sharing, optimizing survival under adverse and nutrient-limited conditions.
Communication and Quorum Sensing Mechanisms
Planktonic cells communicate primarily through individual signaling molecules, while biofilms utilize complex quorum sensing mechanisms that enable coordinated behavior among densely packed microbial communities. In biofilms, signaling pathways regulate gene expression to control biofilm formation, virulence factors, and resistance traits, enhancing collective survival. Quorum sensing in biofilms involves autoinducers such as acyl-homoserine lactones (AHLs) in Gram-negative bacteria and oligopeptides in Gram-positive bacteria, facilitating synchronized responses to environmental stimuli.
Resistance to Antimicrobial Agents
Planktonic cells, existing as free-floating microorganisms, exhibit lower resistance to antimicrobial agents due to their direct exposure and lack of protective matrix. In contrast, biofilms, structured communities of cells encased in extracellular polymeric substances (EPS), demonstrate significantly enhanced resistance by limiting antimicrobial penetration, promoting gene exchange, and employing efflux pumps. This intrinsic resistance in biofilms contributes to chronic infections and challenges in clinical treatment, necessitating alternative therapeutic strategies.
Clinical Implications: Infection and Treatment Challenges
Planktonic cells, characterized by their free-floating state, exhibit increased susceptibility to antibiotics but contribute to the rapid spread of infections in clinical settings. Biofilms, structured communities of microorganisms adhering to surfaces, present significant treatment challenges due to their enhanced resistance to antimicrobial agents and evasion of host immune responses. This persistent nature of biofilms necessitates more aggressive and prolonged therapeutic strategies, often leading to chronic infections and increased healthcare costs.
Industrial and Environmental Impacts
Planktonic cells in industrial water systems contribute to rapid microbial contamination and corrosion, causing equipment degradation and increased maintenance costs. Biofilms, however, form structured communities on surfaces, enhancing resistance to biocides and leading to persistent fouling challenges in pipelines and cooling towers. In environmental contexts, biofilms promote nutrient cycling and contaminant degradation, whereas planktonic cells are more susceptible to environmental stress, affecting microbial ecosystem dynamics and pollutant biotransformation rates.
Future Directions for Research and Therapeutic Approaches
Future research on planktonic cells versus biofilm focuses on elucidating the molecular mechanisms driving biofilm formation and antibiotic resistance to develop targeted therapies. Advancements in nanotechnology and quorum sensing inhibitors show promise in disrupting biofilm integrity and enhancing drug delivery. Innovative strategies combining antimicrobial agents with biofilm-dispersing compounds hold potential for more effective treatments against chronic infections.
Planktonic cells Infographic
 libterm.com
libterm.com