Conventional fossil fuels such as coal, oil, and natural gas remain dominant energy sources due to their high energy density and established infrastructure. Their extraction and combustion contribute significantly to greenhouse gas emissions, driving climate change and environmental degradation. Explore the rest of the article to understand the challenges and future prospects surrounding your energy choices.
Table of Comparison
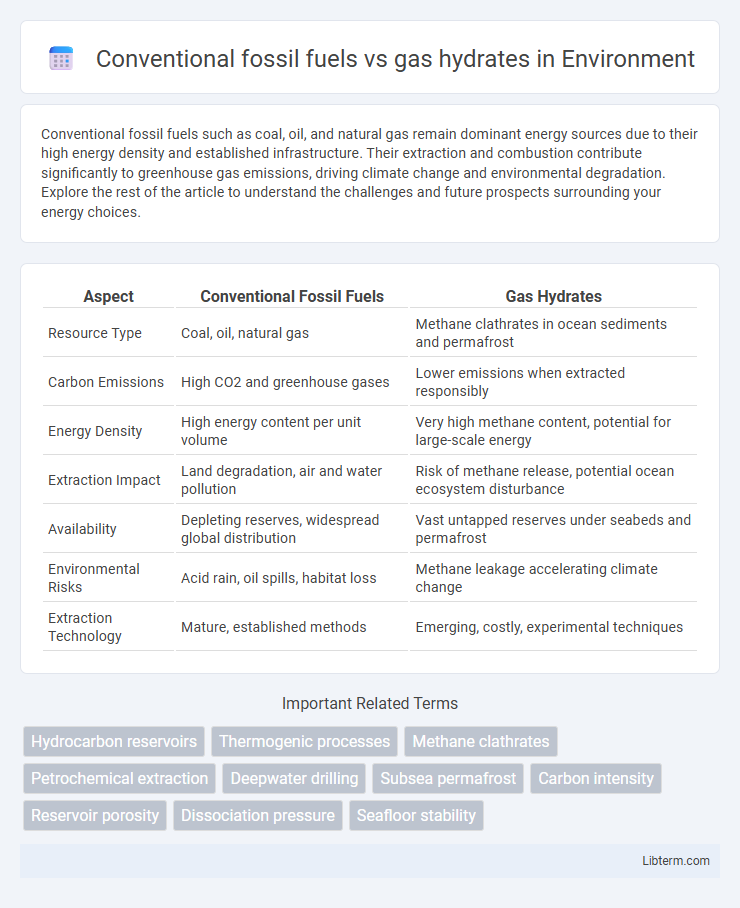
| Aspect | Conventional Fossil Fuels | Gas Hydrates |
|---|---|---|
| Resource Type | Coal, oil, natural gas | Methane clathrates in ocean sediments and permafrost |
| Carbon Emissions | High CO2 and greenhouse gases | Lower emissions when extracted responsibly |
| Energy Density | High energy content per unit volume | Very high methane content, potential for large-scale energy |
| Extraction Impact | Land degradation, air and water pollution | Risk of methane release, potential ocean ecosystem disturbance |
| Availability | Depleting reserves, widespread global distribution | Vast untapped reserves under seabeds and permafrost |
| Environmental Risks | Acid rain, oil spills, habitat loss | Methane leakage accelerating climate change |
| Extraction Technology | Mature, established methods | Emerging, costly, experimental techniques |
Introduction to Conventional Fossil Fuels and Gas Hydrates
Conventional fossil fuels, including coal, oil, and natural gas, are organic hydrocarbon deposits formed over millions of years from decomposed ancient biological matter, providing the majority of global energy consumption. Gas hydrates, crystalline substances composed of methane molecules trapped within water ice, are found in marine sediments and permafrost regions, representing a vast, yet largely untapped, potential energy resource. Unlike conventional fossil fuels, gas hydrates offer a higher energy density and could play a significant role in future energy strategies amid rising demand and environmental concerns.
Geological Formation Processes
Conventional fossil fuels such as coal, oil, and natural gas form over millions of years from the decomposition of organic matter subjected to heat and pressure in sedimentary basins. Gas hydrates, also known as methane clathrates, are crystalline structures formed when methane gas becomes trapped within ice lattices under specific low temperature and high pressure conditions, typically found in deep marine sediments and permafrost regions. The geological formation of gas hydrates involves microbial methanogenesis or thermogenic processes, followed by methane migration into suitable porous sediments where hydrate stability zones exist.
Global Distribution and Availability
Conventional fossil fuels such as coal, oil, and natural gas are concentrated in well-explored sedimentary basins primarily in the Middle East, North America, Russia, and parts of Asia, with proven reserves abundant but geographically limited. Gas hydrates, ice-like crystalline structures containing methane, are widely distributed along continental margins, permafrost regions, and deep-sea sediments, representing an extensive but currently less accessible methane resource. The global availability of gas hydrates potentially exceeds that of conventional fossil fuels, positioning them as a significant future energy resource subject to technological and environmental development.
Extraction Techniques and Technologies
Extraction of conventional fossil fuels such as coal, oil, and natural gas relies on established drilling, mining, and hydraulic fracturing technologies with mature infrastructure supporting global energy markets. Gas hydrate extraction employs emerging techniques like depressurization, thermal stimulation, and chemical injection to dissociate solid methane clathrates trapped in ocean sediments or permafrost, presenting complex challenges in stability control and environmental risk mitigation. Advanced technologies integrating seismic monitoring, remote-operated submersibles, and methane capture systems are pivotal for enabling sustainable and economically viable gas hydrate exploitation compared to conventional fossil fuel extraction.
Energy Density and Efficiency Comparison
Conventional fossil fuels like coal, oil, and natural gas have high energy densities typically ranging from 24 to 48 MJ/kg, offering efficient combustion and well-established extraction technologies. Gas hydrates store methane within ice-like structures under seabed conditions and possess energy densities comparable to liquid natural gas at about 55 MJ/kg, but face significant challenges in efficient and cost-effective extraction. While fossil fuels benefit from mature infrastructure and high combustion efficiency around 40%, gas hydrates represent a vast untapped methane resource with potential for higher energy yield per volume but require advancements in safe and scalable recovery methods to match conventional fuel efficiencies.
Environmental Impacts and Risks
Conventional fossil fuels such as coal, oil, and natural gas release significant greenhouse gases and pollutants during extraction and combustion, contributing to air pollution, climate change, and ecosystem damage. Gas hydrates, potential alternative energy sources trapped in ocean sediments and permafrost, pose risks of methane leakage--a potent greenhouse gas--and disturbances to fragile marine environments during extraction. The environmental impacts of exploiting gas hydrates remain less understood but could exacerbate climate change if methane release is not carefully managed.
Economic Viability and Market Potential
Conventional fossil fuels dominate the global energy market due to established extraction technologies and extensive infrastructure, ensuring cost-effective production and widespread availability. Gas hydrates contain vast methane reserves with potential economic value, but high extraction costs, technological challenges, and environmental risks currently limit their market viability. Advancements in hydrate extraction methods and favorable regulatory policies could enhance their competitiveness and unlock significant energy market potential.
Role in Transitioning to Clean Energy
Conventional fossil fuels, such as coal, oil, and natural gas, remain dominant energy sources but contribute significantly to greenhouse gas emissions and climate change. Gas hydrates, crystalline ice-like structures containing methane, offer a vast, yet largely untapped, potential for cleaner natural gas extraction with lower carbon intensity compared to coal and oil. Utilizing gas hydrates as a transitional fuel can support the gradual shift from high-emission fossil fuels to renewable energy sources by providing a bridge that reduces overall carbon footprints during the global energy transition.
Regulatory and Safety Considerations
Regulatory frameworks for conventional fossil fuels are well-established, encompassing extensive environmental and safety standards due to decades of use and extensive accident data. Gas hydrates, as an emerging energy resource, face evolving regulatory challenges related to drilling safety, methane leakage risks, and potential environmental impacts on marine ecosystems. Strict international guidelines and advanced monitoring technologies are critical to mitigate hazards and ensure sustainable exploitation of gas hydrate reserves.
Future Prospects and Research Directions
Conventional fossil fuels face declining reserves and increasing environmental concerns, prompting a shift toward alternative energy sources like gas hydrates, which hold vast untapped potential in subsea sediments and permafrost regions. Research focuses on developing efficient extraction technologies, minimizing environmental impact, and understanding gas hydrate stability under changing climate conditions. Future prospects emphasize integrating gas hydrate exploitation with carbon capture and storage (CCS) to create sustainable, low-emission energy solutions.
Conventional fossil fuels Infographic
 libterm.com
libterm.com