Monoterpenes are a class of terpenes consisting of two isoprene units, commonly found in essential oils and responsible for their distinctive aromas and therapeutic properties. These compounds exhibit a wide range of biological activities, including anti-inflammatory, antimicrobial, and antioxidant effects that benefit both health and wellness. Explore the article to discover how monoterpenes can enhance your understanding of natural remedies and their applications.
Table of Comparison
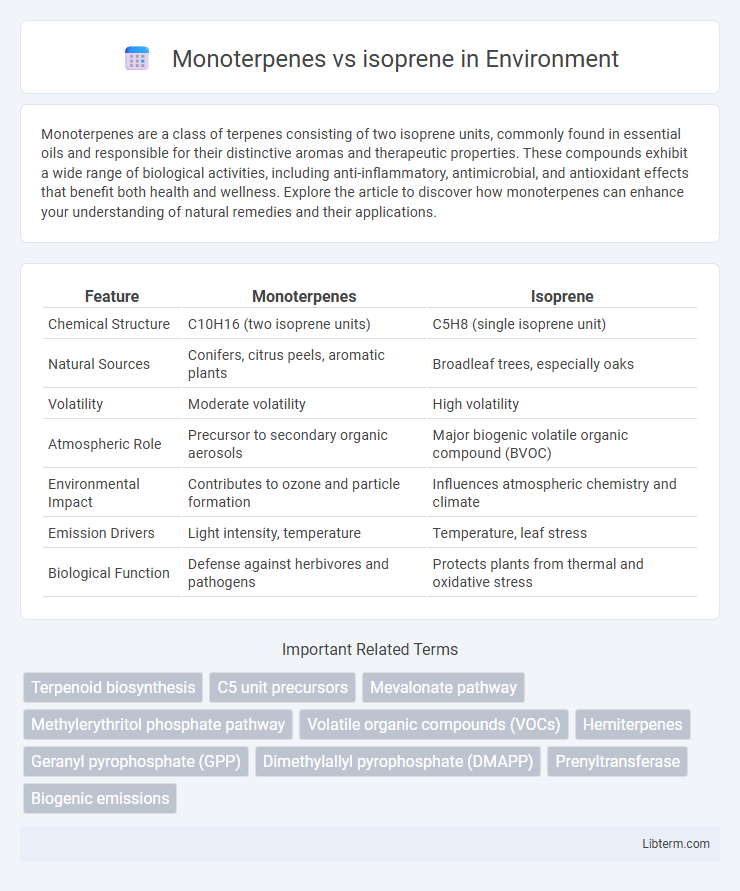
| Feature | Monoterpenes | Isoprene |
|---|---|---|
| Chemical Structure | C10H16 (two isoprene units) | C5H8 (single isoprene unit) |
| Natural Sources | Conifers, citrus peels, aromatic plants | Broadleaf trees, especially oaks |
| Volatility | Moderate volatility | High volatility |
| Atmospheric Role | Precursor to secondary organic aerosols | Major biogenic volatile organic compound (BVOC) |
| Environmental Impact | Contributes to ozone and particle formation | Influences atmospheric chemistry and climate |
| Emission Drivers | Light intensity, temperature | Temperature, leaf stress |
| Biological Function | Defense against herbivores and pathogens | Protects plants from thermal and oxidative stress |
Introduction to Monoterpenes and Isoprene
Monoterpenes are a class of terpenes consisting of two isoprene units, totaling 10 carbon atoms, commonly found in the essential oils of plants and contributing to their aroma and biological activity. Isoprene, a 5-carbon hydrocarbon, serves as the fundamental building block for monoterpenes and other terpenoids, synthesized via the mevalonate and methylerythritol phosphate pathways in plants. The distinction between monoterpenes and isoprene lies in their molecular complexity and biosynthetic roles, with monoterpenes exhibiting diverse structures and functions derived from repetitive isoprene units.
Chemical Structure Comparison
Monoterpenes consist of two isoprene units linked head-to-tail, resulting in a C10H16 molecular formula, whereas isoprene itself is a single C5H8 unit. The chemical structure of monoterpenes features a more complex arrangement with various possible cyclic and acyclic forms, while isoprene remains a simple, reactive diene with two double bonds. These structural differences influence their volatility, stability, and roles in biological and industrial applications.
Biosynthesis Pathways
Monoterpenes are synthesized through the mevalonate (MVA) pathway or the methylerythritol phosphate (MEP) pathway, producing geranyl diphosphate (GPP) as a precursor, while isoprene is primarily formed via the MEP pathway in chloroplasts. The MVA pathway operates in the cytosol and converts acetyl-CoA into isopentenyl diphosphate (IPP), whereas the MEP pathway uses pyruvate and glyceraldehyde-3-phosphate to generate IPP and dimethylallyl diphosphate (DMAPP). Monoterpene synthases then catalyze the conversion of GPP into various monoterpenes, highlighting distinct enzymatic steps differentiating monoterpene and isoprene biosynthesis.
Natural Sources and Distribution
Monoterpenes, composed of two isoprene units, predominantly occur in essential oils of plants such as conifers, citrus fruits, and herbs, contributing to their aromatic properties. Isoprene, a fundamental building block of natural rubber and many biogenic volatile organic compounds, is primarily emitted by broadleaf trees like oaks and poplars in large amounts into the atmosphere. The distribution of monoterpenes is more concentrated in specific plant-exuded resins and oils, while isoprene exhibits widespread atmospheric dispersion due to its gaseous emission from diverse vegetation types.
Ecological Roles and Functions
Monoterpenes, composed of two isoprene units, play crucial roles in plant defense by deterring herbivores and attracting pollinators through their volatile organic compounds. Isoprene, a single five-carbon unit, primarily protects plants from thermal and oxidative stress by stabilizing cell membranes and scavenging reactive oxygen species. Both compounds significantly influence atmospheric chemistry; monoterpenes contribute to aerosol formation, affecting cloud condensation, while isoprene impacts tropospheric ozone and hydroxyl radical levels, thereby shaping ecological interactions.
Industrial and Commercial Applications
Monoterpenes, composed of two isoprene units, are widely used in the industrial production of fragrances, flavors, and solvents due to their strong aromatic properties and volatility. Isoprene, a fundamental building block in synthetic rubber manufacturing, plays a crucial role in producing polyisoprene for automotive tires, adhesives, and elastic materials. Both compounds serve distinct commercial purposes: monoterpenes primarily enhance consumer products, whereas isoprene is essential for durable industrial materials.
Environmental Impact and Emissions
Monoterpenes and isoprene are significant biogenic volatile organic compounds (BVOCs) that impact atmospheric chemistry and air quality. Monoterpenes, emitted mainly by coniferous trees, contribute to secondary organic aerosol formation, influencing cloud condensation nuclei and regional climate regulation. Isoprene emissions, predominantly from deciduous broadleaf trees, react rapidly with atmospheric oxidants, affecting tropospheric ozone formation and thus leading to varied environmental effects on air pollution and human health.
Health Effects and Toxicology
Monoterpenes, composed of two isoprene units, exhibit diverse health effects including anti-inflammatory, antimicrobial, and antioxidant properties, though excessive inhalation may cause respiratory irritation or sensitization. Isoprene itself, a volatile organic compound released naturally by plants and humans, is associated with respiratory tract irritation and potential carcinogenicity upon prolonged exposure to high concentrations. Both compounds impact indoor air quality and human health, necessitating controlled exposure limits to mitigate toxicological risks.
Analytical Methods for Detection
Monoterpenes and isoprene are volatile organic compounds commonly analyzed using gas chromatography-mass spectrometry (GC-MS) due to their distinct molecular structures and volatility. Solid-phase microextraction (SPME) enhances sample preparation by concentrating trace levels of monoterpenes and isoprene from complex matrices before GC-MS analysis. Complementary techniques such as proton transfer reaction-mass spectrometry (PTR-MS) provide real-time, sensitive detection of these compounds in environmental and biological samples.
Future Research and Innovations
Future research on monoterpenes and isoprene is likely to emphasize sustainable biosynthesis pathways leveraging engineered microbial systems to enhance yield and specificity. Innovations in synthetic biology and metabolic engineering could enable tailored monoterpene production for pharmaceuticals, biofuels, and fragrance industries using renewable substrates. Advanced omics technologies and computational modeling are expected to accelerate discovery of novel enzymes and regulatory mechanisms governing isoprene and monoterpene biosynthesis, facilitating efficient industrial-scale applications.
Monoterpenes Infographic
 libterm.com
libterm.com