Heavy metals are dense elements found naturally in the earth's crust, often accumulating in water, soil, and living organisms, posing significant health risks when present in high concentrations. Exposure to heavy metals such as lead, mercury, and cadmium can cause serious conditions including neurological damage and kidney failure, making their detection and mitigation critical. Discover how you can identify sources of heavy metal contamination and protect your health in the rest of this article.
Table of Comparison
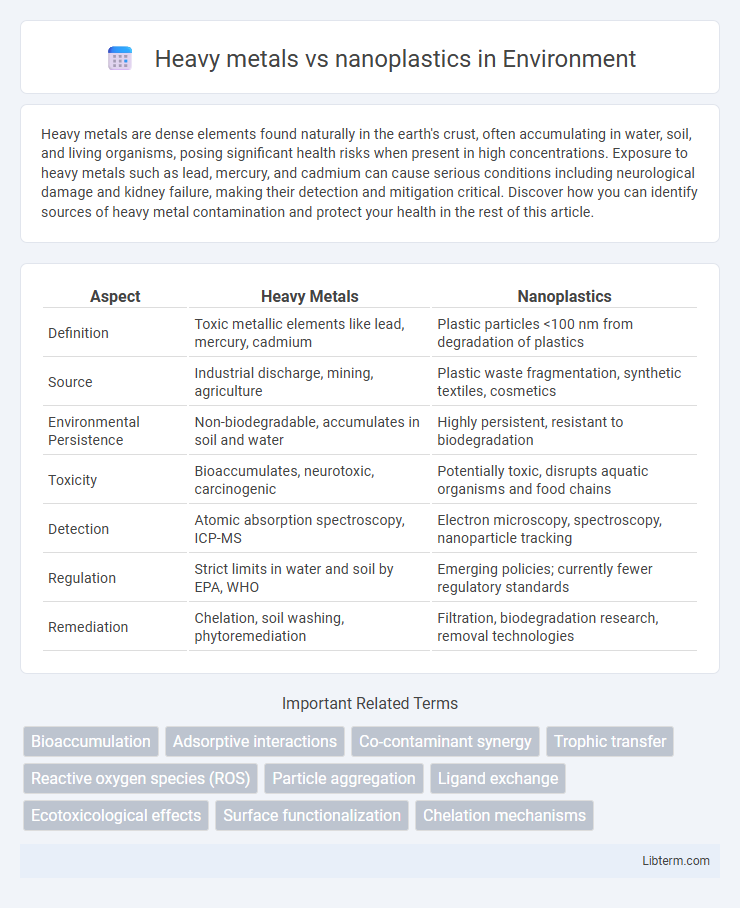
| Aspect | Heavy Metals | Nanoplastics |
|---|---|---|
| Definition | Toxic metallic elements like lead, mercury, cadmium | Plastic particles <100 nm from degradation of plastics |
| Source | Industrial discharge, mining, agriculture | Plastic waste fragmentation, synthetic textiles, cosmetics |
| Environmental Persistence | Non-biodegradable, accumulates in soil and water | Highly persistent, resistant to biodegradation |
| Toxicity | Bioaccumulates, neurotoxic, carcinogenic | Potentially toxic, disrupts aquatic organisms and food chains |
| Detection | Atomic absorption spectroscopy, ICP-MS | Electron microscopy, spectroscopy, nanoparticle tracking |
| Regulation | Strict limits in water and soil by EPA, WHO | Emerging policies; currently fewer regulatory standards |
| Remediation | Chelation, soil washing, phytoremediation | Filtration, biodegradation research, removal technologies |
Introduction to Heavy Metals and Nanoplastics
Heavy metals such as lead, mercury, cadmium, and arsenic are naturally occurring elements with high atomic weight and density that pose significant environmental and health risks due to their toxicity and persistence. Nanoplastics are tiny plastic particles less than 100 nanometers in size, emerging as a critical pollutant from the degradation of larger plastic debris and exhibiting potential for bioaccumulation and toxicological effects in ecosystems. Both heavy metals and nanoplastics contaminate water, soil, and air, challenging conventional remediation techniques and demanding advanced scientific approaches for detection and management.
Sources of Heavy Metals in the Environment
Heavy metals enter the environment primarily through industrial activities, mining, agricultural runoff, and improper waste disposal. Key sources include smelting operations releasing cadmium and lead, fossil fuel combustion emitting mercury, and the use of metal-containing pesticides and fertilizers. These metals persist in soil, water, and air, posing toxic risks to ecosystems and human health compared to the emerging threat of nanoplastics.
Origins and Types of Nanoplastics
Heavy metals such as lead, mercury, and cadmium often originate from industrial discharge, mining activities, and improper waste management, leading to widespread environmental contamination. Nanoplastics primarily stem from the degradation of larger plastic debris, synthetic fibers, and microplastic breakdown through photochemical, mechanical, and biological processes in aquatic and terrestrial ecosystems. Key types of nanoplastics include polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyvinyl chloride (PVC), which differ in polymer composition and environmental persistence, posing varied toxicological risks compared to heavy metals.
Chemical Properties: Heavy Metals vs Nanoplastics
Heavy metals exhibit high atomic mass and density, with distinct metallic bonding that confers unique chemical reactivity, including redox activity and strong affinity for sulfur-containing groups in biological molecules. Nanoplastics are composed of polymeric chains with hydrophobic surfaces and varying functional groups introduced during manufacturing or environmental aging, influencing their adsorption behavior and chemical stability. The chemical inertness of nanoplastics contrasts with the reactive electron configurations of heavy metals, affecting their environmental persistence and interaction with biota.
Environmental Fate and Transport
Heavy metals such as lead, mercury, and cadmium exhibit persistent environmental fate due to their non-degradable nature and tendency to bioaccumulate in soil and aquatic sediments. Nanoplastics, emerging contaminants primarily composed of fragmented plastic polymers smaller than 100 nm, display distinctive transport behaviors influenced by their size, surface charge, and interactions with natural organic matter, facilitating widespread dispersion in water bodies and atmospheric compartments. Both pollutants exhibit complex environmental mobility patterns governed by physicochemical properties and environmental conditions, posing significant challenges for remediation and risk assessment in ecosystems.
Bioaccumulation and Toxicity Mechanisms
Heavy metals such as mercury, lead, and cadmium bioaccumulate in aquatic organisms through direct uptake and trophic transfer, disrupting enzymatic activities and causing oxidative stress at the cellular level. Nanoplastics, due to their nanoscale size and large surface area, penetrate biological membranes more easily, facilitating bioaccumulation and inducing toxicity via inflammation, genotoxicity, and impaired energy metabolism. Both contaminants exhibit synergistic toxicity mechanisms, intensifying oxidative damage and interfering with ion regulation, ultimately threatening ecosystem health and organismal survival.
Human Health Risks: Comparative Analysis
Heavy metals, such as lead, mercury, and cadmium, pose significant human health risks through bioaccumulation, neurotoxicity, and organ damage due to their persistence and heavy bioavailability. Nanoplastics, particles smaller than 100 nanometers, present emerging concerns by potentially crossing biological barriers, inducing oxidative stress, and triggering inflammatory responses, though their long-term effects remain under investigation. Comparative analysis reveals that while heavy metals have well-documented toxicological profiles, nanoplastics introduce complex exposure pathways and synergistic effects that may exacerbate health risks, necessitating integrated assessment frameworks.
Detection and Monitoring Techniques
Detection and monitoring techniques for heavy metals primarily rely on methods such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and X-ray fluorescence (XRF), which provide precise quantification of metal concentrations in environmental samples. Nanoplastics detection involves advanced techniques like dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), and Fourier-transform infrared spectroscopy (FTIR), enabling the characterization of particle size, morphology, and chemical composition at nanoscale levels. Emerging methods integrating spectroscopy and microscopy, coupled with machine learning algorithms, enhance the sensitivity and accuracy of detecting both heavy metals and nanoplastics in complex environmental matrices.
Regulatory Frameworks and Risk Management
Regulatory frameworks for heavy metals, such as mercury and lead, are well-established with specific permissible exposure limits and continuous monitoring protocols enforced by agencies like the EPA and EU REACH. In contrast, nanoplastics face emerging regulatory scrutiny due to limited standardized detection methods and unclear toxicological profiles, prompting precautionary approaches in risk management. Risk management strategies for both contaminants emphasize source control and environmental remediation, but nanoplastics require innovative regulatory updates to address their nanoscale behavior and potential bioaccumulation effectively.
Future Research Directions and Mitigation Strategies
Future research directions on heavy metals and nanoplastics emphasize advanced detection methods and their combined toxicological impacts on ecosystems. Innovative mitigation strategies involve developing bio-based remediation technologies and policy frameworks targeting pollutant source reduction. Strengthening interdisciplinary collaboration enhances understanding of pollutant behavior and effective environmental management solutions.
Heavy metals Infographic
 libterm.com
libterm.com