Sulfate-rich mine drainage significantly impacts water quality by introducing high concentrations of sulfate and heavy metals into the environment, causing contamination of aquatic ecosystems. This type of pollution can lead to acid mine drainage, which further deteriorates soil and water health, posing risks to both wildlife and human populations. Explore the article to understand effective treatment methods and management strategies for protecting your local waterways.
Table of Comparison
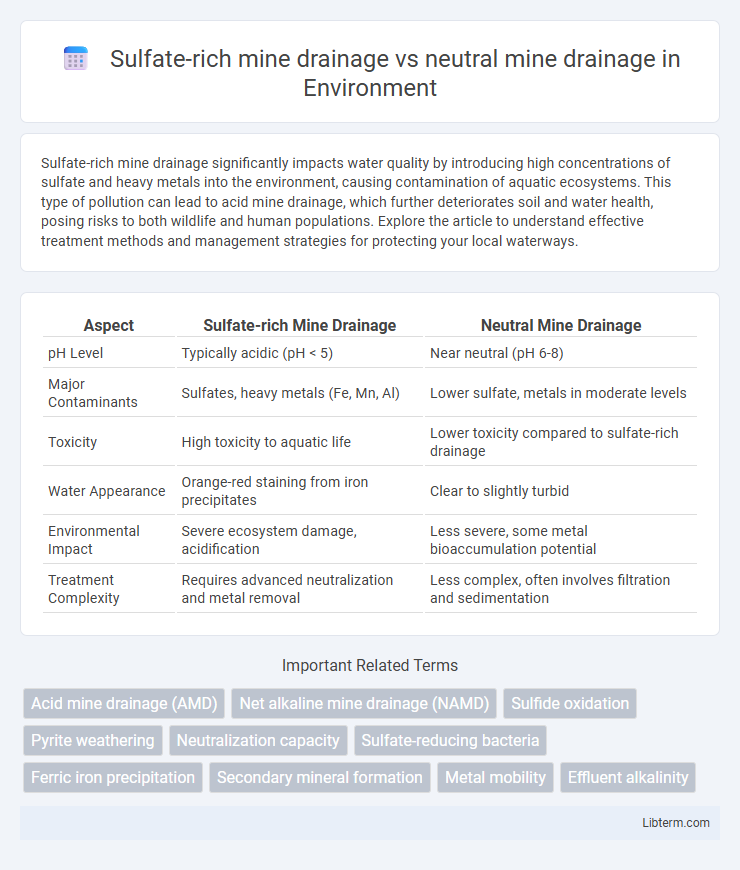
| Aspect | Sulfate-rich Mine Drainage | Neutral Mine Drainage |
|---|---|---|
| pH Level | Typically acidic (pH < 5) | Near neutral (pH 6-8) |
| Major Contaminants | Sulfates, heavy metals (Fe, Mn, Al) | Lower sulfate, metals in moderate levels |
| Toxicity | High toxicity to aquatic life | Lower toxicity compared to sulfate-rich drainage |
| Water Appearance | Orange-red staining from iron precipitates | Clear to slightly turbid |
| Environmental Impact | Severe ecosystem damage, acidification | Less severe, some metal bioaccumulation potential |
| Treatment Complexity | Requires advanced neutralization and metal removal | Less complex, often involves filtration and sedimentation |
Overview of Mine Drainage Types
Sulfate-rich mine drainage typically originates from the oxidation of sulfide minerals, resulting in highly acidic water with elevated sulfate concentrations, metal ions like Fe, Al, and Mn, and a low pH environment that poses significant environmental risks. Neutral mine drainage, often generated in mines with carbonate buffering capacity, maintains a near-neutral pH and contains lower concentrations of dissolved metals and sulfate, reducing its toxicity and treatment complexity. Understanding the chemical composition and pH of these mine drainage types is crucial for developing effective mitigation and remediation strategies tailored to their distinct environmental impacts.
Defining Sulfate-Rich Mine Drainage
Sulfate-rich mine drainage is characterized by high concentrations of sulfate ions (SO4^2-) resulting from the oxidation of sulfide minerals such as pyrite (FeS2) during mining activities, often leading to acidic water conditions. This type of drainage typically contains elevated levels of dissolved metals like iron, aluminum, and heavy metals due to mineral dissolution under acidic conditions. In contrast, neutral mine drainage maintains near-neutral pH levels and generally exhibits lower sulfate concentrations and reduced metal mobilization, often resulting from the presence of buffering minerals like carbonates in the host rock.
Characteristics of Neutral Mine Drainage
Neutral mine drainage typically exhibits a pH range of 6 to 8, minimizing the solubility of heavy metals compared to sulfate-rich mine drainage, which is often highly acidic with pH values below 4. This neutral pH environment results in lower concentrations of dissolved metals and sulfates, reducing the environmental toxicity and metal mobility. Neutral mine drainage commonly originates from mining operations where acid-generating sulfide minerals are limited or well-buffered by surrounding carbonate rocks, leading to more stable water chemistry.
Sources and Formation Processes
Sulfate-rich mine drainage primarily originates from the oxidation of sulfide minerals such as pyrite (FeS2) in coal or metal mines, where exposure to oxygen and water leads to the generation of sulfuric acid. Neutral mine drainage forms when the mining involves carbonate-rich rocks, whose buffering capacity neutralizes the acidity produced by sulfide oxidation, maintaining near-neutral pH levels. The key difference in formation processes lies in the mineralogy of the host rock, with sulfate-rich drainage resulting from intense sulfide oxidation and neutral drainage influenced by carbonate dissolution and acid neutralization reactions.
Geochemical Reactions in Both Drainage Types
Sulfate-rich mine drainage undergoes intense oxidative reactions involving sulfide minerals like pyrite, producing sulfuric acid that lowers pH and mobilizes heavy metals through acid mine drainage processes. Neutral mine drainage maintains near-neutral pH due to buffering reactions with carbonate minerals, leading to precipitation of metal hydroxides and sulfates that reduce metal mobility. These contrasting geochemical reactions dictate the environmental impact and remediation strategies for each drainage type.
Environmental Impacts: Sulfate-Rich vs Neutral
Sulfate-rich mine drainage releases high concentrations of sulfate and dissolved metals, leading to acidification of water bodies and toxicity to aquatic ecosystems through bioaccumulation and sediment contamination. Neutral mine drainage, while less acidic, often contains elevated levels of dissolved metals such as iron and manganese, causing localized metal precipitation and habitat smothering. Both types significantly disrupt aquatic biodiversity and water quality but differ in their chemical interactions and long-term environmental persistence.
Metal Mobility and Contaminant Release
Sulfate-rich mine drainage typically exhibits higher metal mobility due to the acidic conditions that enhance the solubility of heavy metals such as iron, copper, and zinc, leading to increased contaminant release into surrounding environments. Neutral mine drainage generally shows reduced metal mobility as near-neutral pH levels promote the precipitation of metals as hydroxides or carbonates, thereby limiting their bioavailability and transport. Understanding the contrasting geochemical behaviors of sulfate-rich versus neutral mine drainage is critical for developing targeted remediation strategies to mitigate environmental impacts.
Biological Effects and Ecosystem Response
Sulfate-rich mine drainage typically results in elevated concentrations of sulfate ions and heavy metals, leading to toxicity that disrupts microbial communities and reduces biodiversity in aquatic ecosystems. Neutral mine drainage, with pH levels closer to natural water bodies, often causes less acute toxicity but can still introduce metals that bioaccumulate and affect sensitive species over time. Ecosystem responses to sulfate-rich drainage include altered nutrient cycling and diminished primary productivity, while neutral drainage impacts are more subtle but may cause long-term shifts in species composition and food web dynamics.
Treatment and Mitigation Strategies
Sulfate-rich mine drainage requires treatment methods such as lime neutralization, sulfate-reducing bioreactors, and constructed wetlands to precipitate metals and reduce sulfate concentrations effectively. Neutral mine drainage, characterized by near-neutral pH, often necessitates physical treatment like sedimentation and filtration alongside biological approaches that target specific contaminants without extensive pH adjustment. Both types benefit from source control measures, including proper waste rock management and diversion of clean water, to minimize pollutant generation and enhance long-term mitigation outcomes.
Case Studies and Real-World Comparisons
Sulfate-rich mine drainage often exhibits higher acidity and elevated concentrations of metals like iron, aluminum, and manganese compared to neutral mine drainage, impacting local water quality and ecosystem health. Case studies from regions such as the Appalachian coalfields highlight severe acid mine drainage effects causing aquatic toxicity and extensive remediation challenges, whereas neutral mine drainage sites in Scandinavian sulfide mines typically show lower metal solubility and less environmental harm. Real-world comparisons reveal that sulfate-rich drainage requires more intensive treatment methods like lime neutralization and constructed wetlands, while neutral drainage often needs passive systems focused on metal precipitation and sediment control.
Sulfate-rich mine drainage Infographic
 libterm.com
libterm.com