Reduction involves minimizing waste, emissions, and resource consumption to enhance sustainability across industries. Implementing reduction strategies can significantly lower environmental impact while improving operational efficiency and cost savings. Explore the techniques and benefits of reduction to optimize your sustainability efforts in the full article.
Table of Comparison
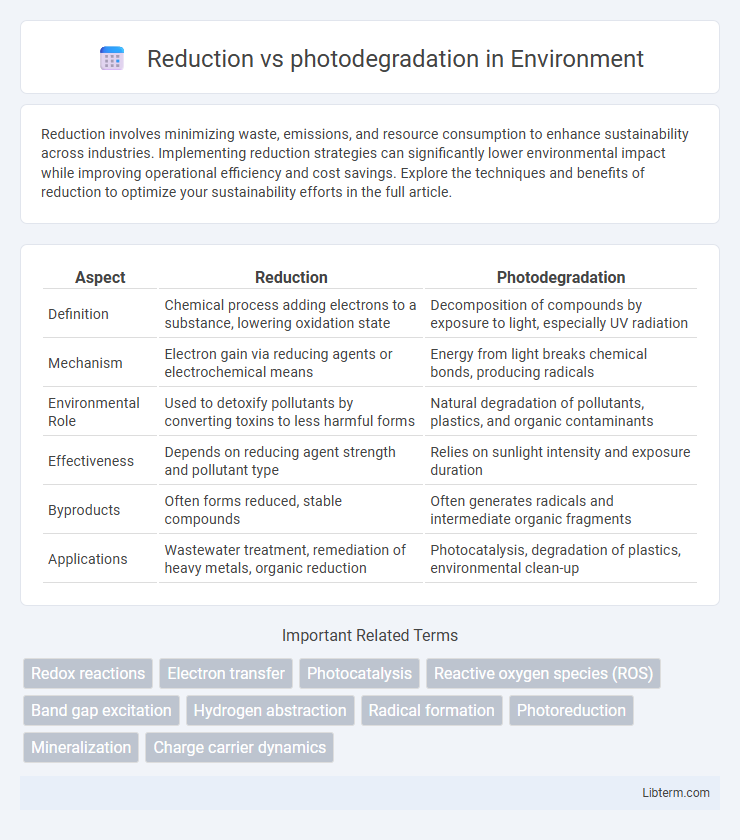
| Aspect | Reduction | Photodegradation |
|---|---|---|
| Definition | Chemical process adding electrons to a substance, lowering oxidation state | Decomposition of compounds by exposure to light, especially UV radiation |
| Mechanism | Electron gain via reducing agents or electrochemical means | Energy from light breaks chemical bonds, producing radicals |
| Environmental Role | Used to detoxify pollutants by converting toxins to less harmful forms | Natural degradation of pollutants, plastics, and organic contaminants |
| Effectiveness | Depends on reducing agent strength and pollutant type | Relies on sunlight intensity and exposure duration |
| Byproducts | Often forms reduced, stable compounds | Often generates radicals and intermediate organic fragments |
| Applications | Wastewater treatment, remediation of heavy metals, organic reduction | Photocatalysis, degradation of plastics, environmental clean-up |
Understanding Reduction and Photodegradation: Definitions
Reduction involves the gain of electrons by a molecule, often leading to a decrease in oxidation state and changes in chemical properties. Photodegradation is the breakdown of compounds through the absorption of light energy, typically resulting in molecular fragmentation or alteration. These processes differ fundamentally as reduction is a redox reaction affecting electron transfer, while photodegradation involves photochemical reactions driven by electromagnetic radiation.
Key Differences Between Reduction and Photodegradation
Reduction involves a chemical process where molecules gain electrons, often facilitated by reducing agents under controlled conditions, whereas photodegradation refers to the breakdown of substances through the absorption of light energy, typically UV radiation. Key differences include the nature of the reaction mechanism--electron transfer in reduction versus photon-induced molecular cleavage in photodegradation--and the environmental factors influencing each, such as the presence of catalysts or specific wavelengths of light. Reduction is commonly used in chemical synthesis and remediation, while photodegradation plays a significant role in environmental processes like pollutant degradation and material aging.
Chemical Mechanisms Involved in Reduction
Reduction involves the gain of electrons by a molecule, resulting in the decrease of its oxidation state through mechanisms such as electron transfer from reducing agents or catalytic hydrogenation. Common reducing agents include metal hydrides, transition metals, and biological reductases, which facilitate mechanisms like nucleophilic attack or electron donation. Unlike photodegradation, where light induces bond cleavage and radical formation, reduction specifically targets electron gain to alter molecular structure and reactivity.
Chemical Mechanisms Involved in Photodegradation
Photodegradation primarily involves the absorption of photons, leading to the excitation of molecules and subsequent generation of reactive species such as singlet oxygen, free radicals, and ions. These reactive intermediates initiate bond cleavage and molecular transformation through processes including photo-oxidation, electron transfer, and energy transfer mechanisms. Unlike reduction, which involves electron gain, photodegradation mechanisms emphasize light-induced bond dissociation facilitated by excited-state chemistry and photochemical reactions.
Common Applications of Reduction in Industry and Environment
Reduction processes are widely utilized in industries such as metal refining, chemical manufacturing, and wastewater treatment to convert compounds into more stable or less toxic forms. In environmental applications, reduction is essential for mitigating pollutants by transforming hazardous substances like nitrates and heavy metals into less harmful materials. This method supports sustainable practices by enabling the recovery of valuable elements and minimizing environmental contamination.
Photodegradation Applications in Pollution Control
Photodegradation harnesses light energy, typically ultraviolet or visible light, to break down pollutants into less harmful substances through photochemical reactions, making it a powerful tool in environmental remediation. This method is widely applied in treating organic contaminants in water and air, such as pesticides, dyes, and volatile organic compounds, by accelerating their decomposition under sunlight or artificial light sources. Advances in photocatalysts like titanium dioxide (TiO2) enhance photodegradation efficiency, promoting sustainable pollution control by enabling in situ pollutant removal without secondary waste generation.
Factors Influencing Reduction Processes
Reduction processes in environmental chemistry are influenced by factors such as redox potential, the presence of electron donors, pH levels, and temperature, which govern the rate and efficiency of contaminant transformation. The availability of suitable reducing agents, such as Fe(II) or sulfides, enhances the electron transfer necessary for reducing pollutants. Environmental conditions including anaerobic status and microbial activity also play critical roles in facilitating or impeding reduction reactions.
Factors Affecting Photodegradation Efficiency
Photodegradation efficiency is influenced by factors such as light wavelength, intensity, and the presence of photosensitizers that activate reactive species. Environmental conditions including temperature, pH, and dissolved oxygen levels also play critical roles in driving or inhibiting photodegradation reactions. Surface area and the chemical structure of pollutants determine their susceptibility to photodegradation compared to reduction processes.
Environmental Impact: Reduction vs Photodegradation
Reduction processes often lead to the transformation of pollutants into less harmful substances, significantly lowering environmental toxicity and promoting safer ecosystems. Photodegradation utilizes light energy to break down contaminants, accelerating pollutant removal but sometimes producing intermediate byproducts that may still pose ecological risks. Evaluating the environmental impact requires assessing the persistence, toxicity, and bioaccumulation potential of degradation products formed through either reduction or photodegradation pathways.
Future Trends in Reduction and Photodegradation Technologies
Future trends in reduction and photodegradation technologies emphasize the development of advanced catalysts with enhanced selectivity and efficiency under visible light. Integration of nanomaterials such as graphene oxide and metal-organic frameworks (MOFs) is driving improvements in photocatalytic activity and stability. Emerging research also explores hybrid systems combining reduction and photodegradation processes to maximize pollutant removal and energy conversion efficiency.
Reduction Infographic
 libterm.com
libterm.com