Molarity measures the concentration of a solution in moles of solute per liter of solution, providing a clear quantitative assessment. This unit is essential for preparing chemical solutions accurately and calculating reaction stoichiometry. Explore the rest of the article to understand how molarity influences laboratory practices and everyday applications.
Table of Comparison
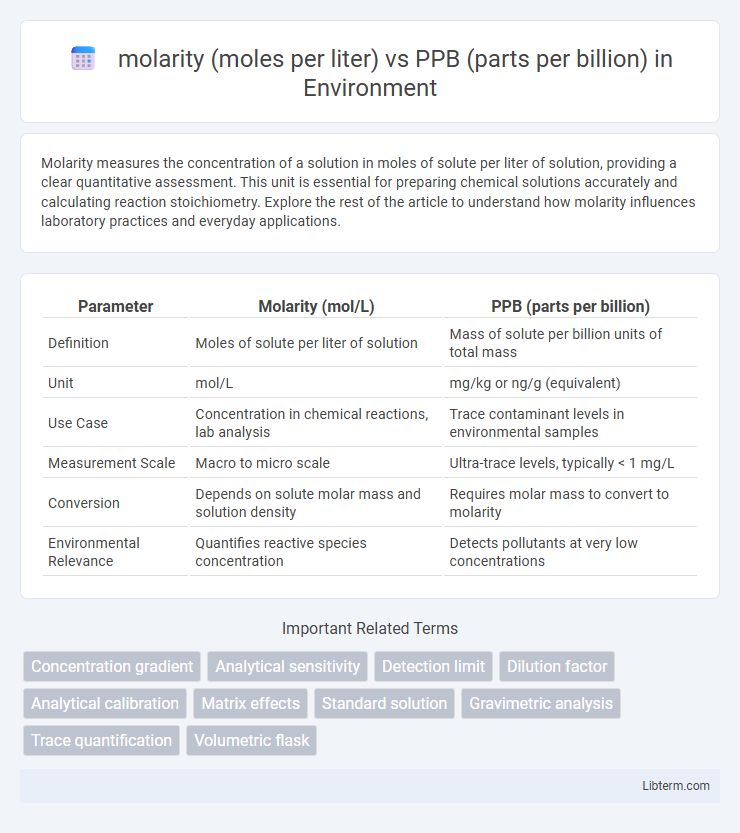
| Parameter | Molarity (mol/L) | PPB (parts per billion) |
|---|---|---|
| Definition | Moles of solute per liter of solution | Mass of solute per billion units of total mass |
| Unit | mol/L | mg/kg or ng/g (equivalent) |
| Use Case | Concentration in chemical reactions, lab analysis | Trace contaminant levels in environmental samples |
| Measurement Scale | Macro to micro scale | Ultra-trace levels, typically < 1 mg/L |
| Conversion | Depends on solute molar mass and solution density | Requires molar mass to convert to molarity |
| Environmental Relevance | Quantifies reactive species concentration | Detects pollutants at very low concentrations |
Understanding Molarity: Definition and Calculation
Molarity, expressed in moles per liter (mol/L), quantifies the concentration of a solute in a solution by indicating the number of moles of solute dissolved in one liter of solvent. The calculation involves dividing the amount of solute in moles by the total volume of the solution in liters, providing a direct measure of chemical concentration essential for stoichiometric analysis. Unlike parts per billion (PPB), which measures the mass of solute per billion units of solution mass or volume, molarity offers a molar-based concentration critical for reaction-based chemistry applications.
What Does PPB (Parts Per Billion) Mean?
PPB (parts per billion) is a unit of concentration that measures the mass of a substance per billion units of total mass, commonly used to express extremely low concentrations in water or air. Unlike molarity, which quantifies concentration in moles per liter, PPB indicates the ratio of a contaminant's mass to the total mass, making it essential for environmental analysis of trace pollutants. Understanding PPB allows precise detection of contaminants at nanogram-per-liter levels, critical for assessing environmental and health safety standards.
Comparing Units: Molarity vs PPB Explained
Molarity measures concentration as moles of solute per liter of solution, providing a precise quantification of chemical species in a given volume. Parts per billion (PPB) express concentration based on the ratio of mass or volume of a substance to one billion parts of the total mixture, commonly used for trace contaminants. Converting between molarity and PPB depends on the molecular weight of the solute and the density of the solution, making understanding both units critical for accurate chemical analysis and environmental monitoring.
Conversion Methods Between Molarity and PPB
Conversion between molarity (moles per liter) and parts per billion (PPB) requires knowledge of the solute's molar mass and solution density. To convert molarity to PPB, multiply the molarity by the molar mass (g/mol) and 10^9, assuming the density of the solution is close to 1 g/mL. Conversely, converting PPB to molarity involves dividing the PPB value by the molar mass and 10^9, adjusted by the solution's density if significant.
Applications of Molarity in Laboratory Settings
Molarity (moles per liter) is essential in laboratory settings for accurately preparing solutions with precise concentrations, critical for chemical reactions and titrations. It allows chemists to quantify reactants, ensuring stoichiometric balance in experiments, which is vital in pharmaceutical and analytical chemistry. PPB (parts per billion) is generally used for measuring trace contaminants in environmental analysis, whereas molarity focuses on controlled solution concentrations in research and industrial laboratories.
PPB in Environmental and Analytical Chemistry
PPB (parts per billion) measures trace concentrations essential for detecting pollutants in environmental and analytical chemistry, especially for assessing contaminants like heavy metals and organic compounds in water and air. Molarity (moles per liter) quantifies solute concentration in chemical reactions but is less practical for ultra-trace detection where detecting nanogram per liter levels is crucial. PPB units facilitate precise monitoring of environmental pollutants, ensuring compliance with regulatory standards and safeguarding public health.
Sensitivity and Suitability: When to Use Each Unit
Molarity measures concentration in moles per liter, offering precise quantification of solutes in chemical reactions and laboratory settings where exact mole ratios are critical for stoichiometry. Parts per billion (PPB) is ideal for detecting extremely low concentrations of contaminants in environmental monitoring and trace analysis, providing heightened sensitivity for substances present at nanogram-per-liter levels. Use molarity when exact chemical concentrations are necessary for reaction control, while PPB is more suitable for ultra-trace detection where sensitivity to minute quantities is crucial.
Real-World Examples of Molarity and PPB Calculations
Molarity, expressed in moles per liter (mol/L), quantifies solute concentration in solutions such as a 1 M sodium chloride solution containing 58.44 grams of NaCl dissolved per liter of water. Parts per billion (PPB) measures trace contaminants, like 10 PPB arsenic in groundwater indicating 10 micrograms of arsenic per liter, critical for environmental safety assessments. Calculating molarity involves dividing moles of solute by liters of solution, whereas PPB compares the mass of solute to the total mass or volume, useful in fields from chemistry labs to environmental monitoring.
Common Mistakes in Interpreting Concentration Units
Confusing molarity (moles per liter) with parts per billion (PPB) often leads to errors in concentration interpretation due to their fundamentally different bases--molarity measures the number of moles of solute per liter of solution, while PPB quantifies the mass of solute per billion units of total solution mass or volume. A common mistake is assuming direct equivalence without accounting for molar mass, solution density, or temperature, which significantly affect the conversion between these units. Proper conversion requires accurate molecular weights and system conditions to avoid errors in chemical calculations or environmental measurements.
Choosing the Right Unit for Accurate Chemical Analysis
Molarity (moles per liter) provides a precise concentration measure ideal for reactions involving known quantities of solutes, ensuring accurate stoichiometric calculations in chemical analysis. Parts per billion (PPB) is crucial for detecting trace contaminants at extremely low concentrations, commonly used in environmental monitoring and toxicology. Selecting the appropriate unit depends on the analytical context: molarity fits quantitative reaction settings, while PPB is best for ultra-trace level detection and regulatory compliance.
molarity (moles per liter) Infographic
 libterm.com
libterm.com