Particulate matter consists of tiny particles suspended in the air, which can penetrate your respiratory system and cause serious health problems such as asthma and cardiovascular disease. These particles arise from various sources including vehicle emissions, industrial processes, and natural phenomena like wildfires and dust storms. Discover more about the impact of particulate matter and how you can protect yourself throughout the rest of the article.
Table of Comparison
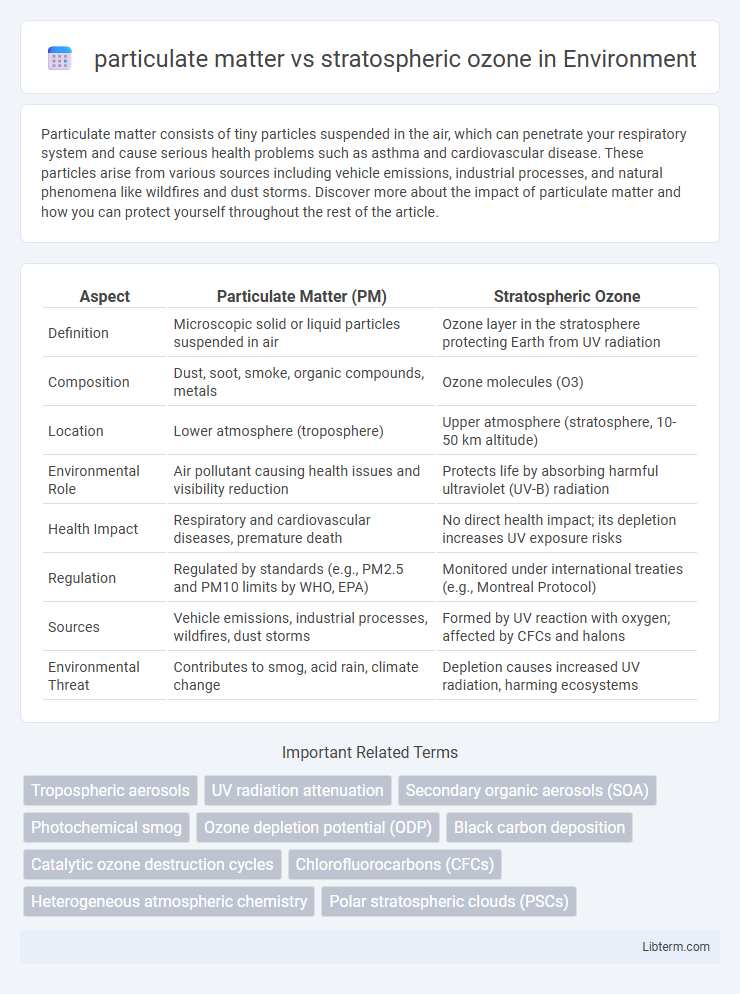
| Aspect | Particulate Matter (PM) | Stratospheric Ozone |
|---|---|---|
| Definition | Microscopic solid or liquid particles suspended in air | Ozone layer in the stratosphere protecting Earth from UV radiation |
| Composition | Dust, soot, smoke, organic compounds, metals | Ozone molecules (O3) |
| Location | Lower atmosphere (troposphere) | Upper atmosphere (stratosphere, 10-50 km altitude) |
| Environmental Role | Air pollutant causing health issues and visibility reduction | Protects life by absorbing harmful ultraviolet (UV-B) radiation |
| Health Impact | Respiratory and cardiovascular diseases, premature death | No direct health impact; its depletion increases UV exposure risks |
| Regulation | Regulated by standards (e.g., PM2.5 and PM10 limits by WHO, EPA) | Monitored under international treaties (e.g., Montreal Protocol) |
| Sources | Vehicle emissions, industrial processes, wildfires, dust storms | Formed by UV reaction with oxygen; affected by CFCs and halons |
| Environmental Threat | Contributes to smog, acid rain, climate change | Depletion causes increased UV radiation, harming ecosystems |
Introduction to Particulate Matter and Stratospheric Ozone
Particulate matter (PM) consists of tiny solid and liquid particles suspended in the air, varying in size from PM10 to PM2.5, which significantly affect air quality and human health. Stratospheric ozone forms a layer approximately 15 to 35 kilometers above Earth's surface, playing a critical role in absorbing harmful ultraviolet radiation. Understanding the distinct properties and environmental impacts of particulate matter and stratospheric ozone is essential for air pollution management and climate protection strategies.
Chemical Composition: Particulate Matter vs Stratospheric Ozone
Particulate matter primarily consists of a mixture of solid particles and liquid droplets, including organic compounds, metals, sulfates, nitrates, and elemental carbon, varying in size from PM10 to PM2.5. Stratospheric ozone is a triatomic molecule (O3) formed by the interaction of molecular oxygen (O2) with ultraviolet (UV) radiation, playing a crucial role in absorbing harmful UV-B rays from the sun. Unlike particulate matter, stratospheric ozone's chemical composition is uniform and purely molecular, whereas particulate matter is chemically diverse and heterogeneous.
Sources and Formation of Particulate Matter
Particulate matter (PM) forms from various sources including combustion processes, industrial emissions, construction activities, and natural sources like wildfires and dust storms. These particles originate through primary emissions or secondary formation when gaseous pollutants such as sulfur dioxide (SO2), nitrogen oxides (NOx), and volatile organic compounds (VOCs) undergo chemical reactions in the atmosphere. Unlike stratospheric ozone, which forms from oxygen molecules under UV radiation, particulate matter is a mixture of solid and liquid particles with diverse compositions influenced by local emission sources and atmospheric conditions.
Formation and Distribution of Stratospheric Ozone
Stratospheric ozone forms primarily through the photodissociation of molecular oxygen (O2) by ultraviolet (UV) light, creating atomic oxygen that subsequently reacts with O2 to produce ozone (O3). This ozone layer is concentrated between 15 to 35 kilometers above the Earth's surface, where UV radiation intensity and oxygen concentration optimize its formation. Unlike particulate matter, which originates from combustion and industrial activities and distributes unevenly in the troposphere, stratospheric ozone is a naturally occurring atmospheric component critical for blocking harmful UV radiation.
Environmental Impacts of Particulate Matter
Particulate matter (PM) significantly degrades air quality by penetrating respiratory systems, leading to increased incidence of cardiovascular and respiratory diseases. Unlike stratospheric ozone, which protects life by absorbing harmful UV radiation, particulate matter contributes directly to atmospheric pollution and visibility reduction. PM also influences climate change by altering radiation balance and acting as cloud condensation nuclei, thereby affecting weather patterns and ecosystems.
Role of Stratospheric Ozone in UV Protection
Stratospheric ozone plays a critical role in absorbing the majority of the sun's harmful ultraviolet (UV) radiation, particularly UV-B and UV-C rays, thereby protecting living organisms from DNA damage and skin cancers. Unlike particulate matter, which primarily affects air quality and respiratory health at ground level, stratospheric ozone functions as a protective shield high in the atmosphere, significantly reducing UV radiation reaching the Earth's surface. This natural UV filtration provided by the ozone layer is essential for maintaining ecological balance and preventing increased biological harm caused by excessive UV exposure.
Health Effects: Particulate Matter vs Stratospheric Ozone Depletion
Exposure to particulate matter (PM) poses significant health risks, including respiratory and cardiovascular diseases, due to fine particles penetrating deep into the lungs and bloodstream. Stratospheric ozone depletion increases ultraviolet (UV) radiation reaching Earth's surface, leading to higher rates of skin cancer, cataracts, and impaired immune function. While particulate matter affects human health through direct inhalation, ozone layer depletion impacts health indirectly by altering UV exposure levels.
Regulatory Measures and Air Quality Standards
Regulatory measures targeting particulate matter (PM) involve strict emissions limits for PM2.5 and PM10 set by agencies such as the EPA under the National Ambient Air Quality Standards (NAAQS) to control sources including industrial activities, vehicle exhaust, and combustion processes. Stratospheric ozone protection is governed by international agreements like the Montreal Protocol, which mandates the phase-out of ozone-depleting substances (ODS) such as CFCs to prevent ozone layer depletion and reduce harmful UV radiation. Both frameworks emphasize continuous monitoring and enforcement to ensure compliance, improve air quality, and protect public health and the environment.
Monitoring and Measurement Techniques
Monitoring particulate matter (PM) relies on advanced air quality sensors such as beta attenuation monitors and optical particle counters that provide real-time concentration data of PM2.5 and PM10 sizes. Stratospheric ozone measurement utilizes satellite-based remote sensing instruments like the Ozone Monitoring Instrument (OMI) and ground-based spectrophotometers, including Dobson and Brewer spectrophotometers, to track ozone layer thickness and distribution globally. Combining in-situ PM sampling with satellite ozone data enhances atmospheric studies by correlating surface pollution levels with stratospheric ozone variations.
Future Challenges and Solutions
Future challenges in managing particulate matter and stratospheric ozone revolve around balancing emission reductions and climate change mitigation. Advanced satellite monitoring and atmospheric modeling can enhance understanding of pollutant interactions and ozone depletion mechanisms. Innovative solutions include deploying low-emission technologies, improving air quality standards, and investing in geoengineering research to protect the ozone layer while reducing particulate pollution.
particulate matter Infographic
 libterm.com
libterm.com