Carbon dioxide is a colorless, odorless gas that plays a crucial role in Earth's carbon cycle and climate regulation. It is emitted through natural processes like respiration and volcanic activity, as well as human activities such as burning fossil fuels, contributing to global warming. Discover how understanding carbon dioxide's impact can help you make informed decisions about environmental sustainability in the rest of this article.
Table of Comparison
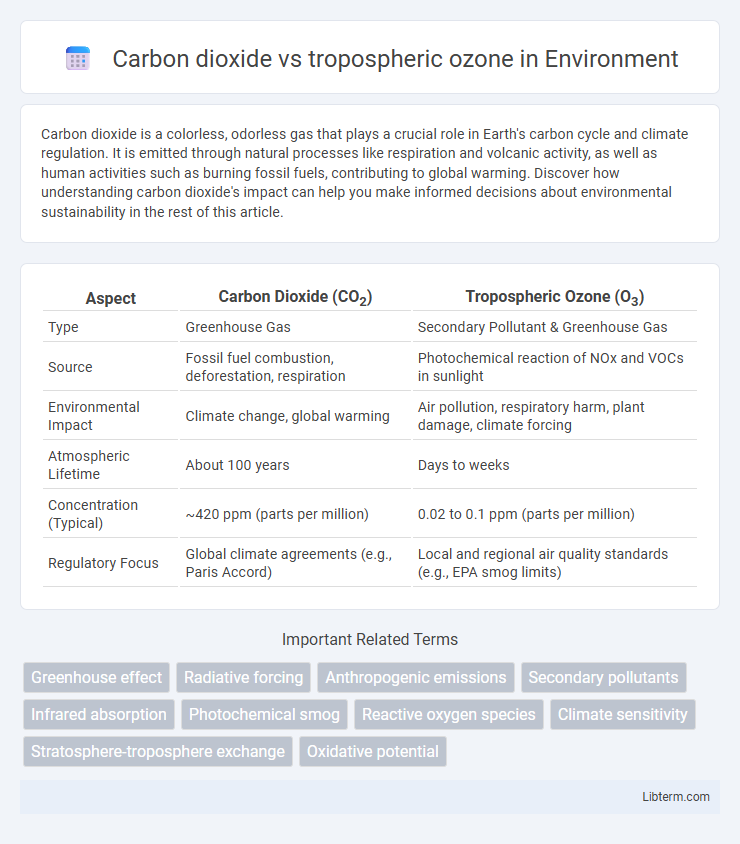
| Aspect | Carbon Dioxide (CO2) | Tropospheric Ozone (O3) |
|---|---|---|
| Type | Greenhouse Gas | Secondary Pollutant & Greenhouse Gas |
| Source | Fossil fuel combustion, deforestation, respiration | Photochemical reaction of NOx and VOCs in sunlight |
| Environmental Impact | Climate change, global warming | Air pollution, respiratory harm, plant damage, climate forcing |
| Atmospheric Lifetime | About 100 years | Days to weeks |
| Concentration (Typical) | ~420 ppm (parts per million) | 0.02 to 0.1 ppm (parts per million) |
| Regulatory Focus | Global climate agreements (e.g., Paris Accord) | Local and regional air quality standards (e.g., EPA smog limits) |
Introduction to Carbon Dioxide and Tropospheric Ozone
Carbon dioxide (CO2) is a greenhouse gas primarily produced by fossil fuel combustion, deforestation, and natural processes, playing a critical role in Earth's energy balance and climate regulation. Tropospheric ozone (O3) forms through photochemical reactions involving nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the lower atmosphere, acting both as a pollutant and a secondary greenhouse gas with harmful effects on human health and vegetation. The contrasting sources and roles of CO2 and tropospheric ozone highlight their interconnected impacts on atmospheric chemistry and global warming.
Chemical Properties and Atmospheric Behavior
Carbon dioxide (CO2) is a stable, non-reactive greenhouse gas with a linear molecular structure, characterized by strong double bonds between carbon and oxygen atoms, making it less reactive in the troposphere. Tropospheric ozone (O3) is a highly reactive, triatomic molecule with a bent structure, acting as a powerful oxidant and playing a critical role in atmospheric chemistry by participating in photochemical reactions. While CO2 primarily accumulates and persists, influencing long-term climate forcing, tropospheric ozone exhibits variable concentrations driven by sunlight and precursor emissions, significantly impacting air quality and short-term radiative forcing.
Primary Sources and Emission Pathways
Carbon dioxide primarily originates from fossil fuel combustion, deforestation, and industrial processes, with emissions entering the atmosphere mainly through direct release during energy production and land-use changes. Tropospheric ozone, unlike CO2, is not emitted directly but forms through photochemical reactions involving precursors such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) released from vehicles, power plants, and chemical solvents. These distinct emission pathways highlight carbon dioxide's role as a long-lived greenhouse gas from primary sources, while tropospheric ozone serves as a secondary pollutant influenced by complex atmospheric chemistry.
Global Distribution Patterns
Carbon dioxide (CO2) exhibits a relatively uniform global distribution due to its long atmospheric lifetime and efficient mixing in the troposphere, with concentrations slightly higher in the Northern Hemisphere because of anthropogenic emissions. Tropospheric ozone (O3), in contrast, shows highly variable spatial distribution influenced by local precursor emissions, photochemical activity, and atmospheric transport, often peaking in urban and industrial regions and decreasing in remote areas. Seasonal and diurnal fluctuations in ozone levels are pronounced, reflecting sensitivity to sunlight and temperature, whereas CO2 increases steadily with minor seasonal variation.
Roles in Climate Change and Warming Potential
Carbon dioxide (CO2) is the primary greenhouse gas driving long-term climate change due to its high atmospheric concentration and long lifetime, significantly contributing to global warming by trapping infrared radiation. Tropospheric ozone (O3) acts as a potent short-lived greenhouse gas with a warming potential approximately 1,000 times greater than CO2 on a per-molecule basis, intensifying climate change over shorter timescales. Both gases influence radiative forcing, but CO2's persistent presence makes it the dominant factor in sustained temperature rise, whereas tropospheric ozone impacts regional climate and air quality with notable feedback on the carbon cycle.
Impacts on Human Health
Carbon dioxide primarily contributes to climate change, indirectly affecting human health through increased heatwaves and air pollution, while tropospheric ozone directly harms respiratory function by causing inflammation and exacerbating asthma, bronchitis, and other chronic lung diseases. Elevated ozone levels are linked to increased hospital admissions and premature mortality due to impaired lung capacity and cardiovascular stress. Unlike carbon dioxide, which is colorless and odorless, tropospheric ozone is a potent oxidant with immediate detrimental effects on airway tissues.
Effects on Ecosystems and Vegetation
Carbon dioxide (CO2) contributes to global warming by enhancing the greenhouse effect, which alters ecosystems through shifts in temperature and precipitation patterns, potentially benefiting plant growth due to increased photosynthesis but also causing stress in heat-sensitive species. Tropospheric ozone (O3), a harmful pollutant, damages vegetation by penetrating leaf tissues, impairing photosynthesis, reducing crop yields, and increasing plant susceptibility to diseases and environmental stress. While elevated CO2 can stimulate plant growth, tropospheric ozone exposure often counteracts these benefits by causing oxidative stress and visible injury, leading to reduced biomass and ecosystem productivity.
Monitoring and Measurement Techniques
Monitoring carbon dioxide involves high-precision infrared gas analyzers and satellite remote sensing platforms such as NASA's OCO-2 to capture real-time atmospheric concentrations and global distribution patterns. Tropospheric ozone measurement relies on ground-based UV absorption photometers and ozonesondes deployed via weather balloons, providing vertical profiles and localized ozone concentration data critical for air quality assessments. Integration of these data sources through atmospheric modeling enhances understanding of greenhouse gas dynamics and ozone formation processes at various spatial and temporal scales.
Mitigation Strategies and Policy Approaches
Carbon dioxide mitigation primarily involves decarbonizing energy sources through renewable technologies and implementing carbon pricing mechanisms like carbon taxes and cap-and-trade systems. Tropospheric ozone reduction strategies focus on controlling precursor emissions such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) via stricter vehicle emission standards and industrial regulations. Policy approaches integrate atmospheric monitoring and cross-sector cooperation to optimize reductions in both greenhouse gases and ozone precursors, enhancing overall air quality and climate benefits.
Future Trends and Research Directions
Future trends indicate a growing emphasis on understanding the synergistic effects of carbon dioxide and tropospheric ozone on climate change and air quality. Research directions prioritize the development of advanced atmospheric models to predict their interactive impacts on greenhouse gas dynamics and ecosystem health. Emerging studies also focus on mitigation strategies that simultaneously address CO2 emissions and ozone precursors to enhance environmental and public health outcomes.
Carbon dioxide Infographic
 libterm.com
libterm.com