Isobar is a global digital marketing agency specializing in creating innovative brand experiences and driving customer engagement through technology and creativity. Leveraging data-driven strategies and cutting-edge digital solutions, Isobar helps businesses enhance their online presence and achieve measurable growth. Discover how Isobar can transform your brand by reading the rest of the article.
Table of Comparison
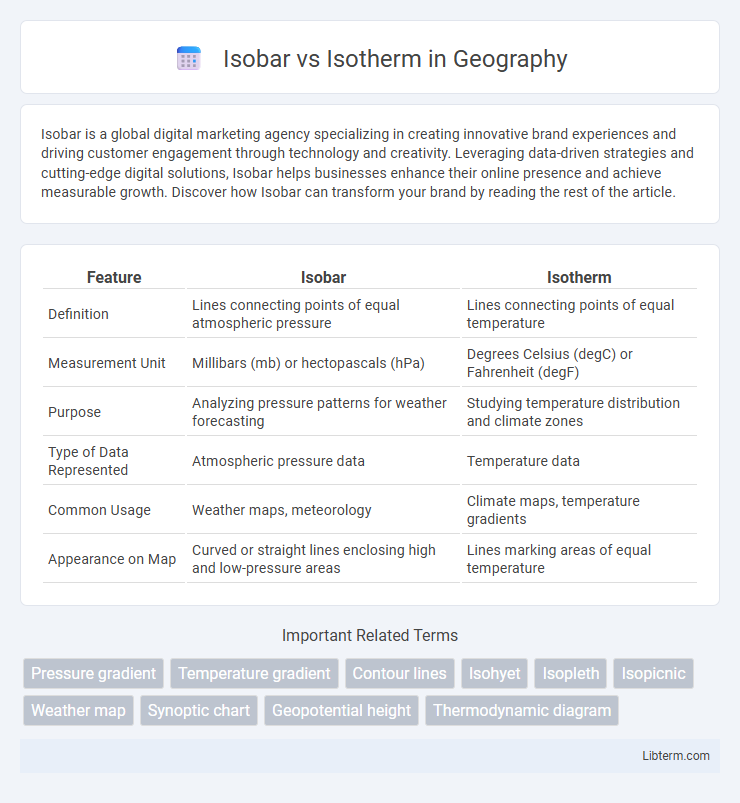
| Feature | Isobar | Isotherm |
|---|---|---|
| Definition | Lines connecting points of equal atmospheric pressure | Lines connecting points of equal temperature |
| Measurement Unit | Millibars (mb) or hectopascals (hPa) | Degrees Celsius (degC) or Fahrenheit (degF) |
| Purpose | Analyzing pressure patterns for weather forecasting | Studying temperature distribution and climate zones |
| Type of Data Represented | Atmospheric pressure data | Temperature data |
| Common Usage | Weather maps, meteorology | Climate maps, temperature gradients |
| Appearance on Map | Curved or straight lines enclosing high and low-pressure areas | Lines marking areas of equal temperature |
Introduction to Isobars and Isotherms
Isobars are lines on a map connecting points of equal atmospheric pressure, essential for understanding weather patterns and predicting storms. Isotherms link points of equal temperature, providing crucial data for climate analysis and temperature distribution studies. Both isobars and isotherms are fundamental tools in meteorology, enabling accurate visualization of pressure systems and thermal variations across geographical areas.
Definition of Isobar
An isobar is a line on a pressure-temperature diagram representing states of equal pressure, crucial for analyzing thermodynamic processes in closed systems. Unlike an isotherm, which connects points of constant temperature, an isobar allows engineers to study phase changes and volume shifts under uniform pressure conditions. Understanding isobars is fundamental in fields like meteorology, chemical engineering, and physical chemistry for predicting system behavior during heating or cooling.
Definition of Isotherm
Isotherms are lines on a graph or map representing constant temperature values, used extensively in thermodynamics and meteorology to analyze temperature distribution. They help visualize thermal gradients and are critical for interpreting heat transfer processes and climate patterns. Unlike isobars, which indicate constant pressure, isotherms specifically highlight temperature uniformity within a given system or environment.
Formation of Isobars on Weather Maps
Isobars are lines on weather maps connecting points of equal atmospheric pressure, essential for identifying high and low-pressure systems and predicting wind patterns. They form through the measurement of pressure variations at different locations, often influenced by temperature differences, altitude, and weather fronts. In contrast, isotherms map areas of equal temperature, serving different meteorological analysis purposes such as temperature distribution and heat wave tracking.
Formation of Isotherms on Weather Maps
Isotherms, representing lines of equal temperature, form on weather maps by connecting data points from temperature readings taken across various geographic locations at a specific time. These lines highlight temperature gradients and help meteorologists identify thermal boundaries, which are crucial for understanding weather patterns and forecasting. Unlike isobars that connect points of equal pressure, isotherms focus solely on temperature distribution, providing insight into heat flux and climate zones.
Key Differences Between Isobars and Isotherms
Isobars are lines on a weather map connecting points of equal atmospheric pressure, while isotherms link points with the same temperature. Isobars help meteorologists analyze pressure systems and forecast wind patterns, whereas isotherms are essential for understanding temperature distribution and climate zones. The primary distinction lies in their measured variables: isobars represent constant pressure, and isotherms indicate constant temperature.
Importance in Meteorology
Isobars and isotherms are essential tools in meteorology for analyzing weather patterns and atmospheric conditions. Isobars, lines connecting points of equal atmospheric pressure, help meteorologists identify high and low-pressure systems crucial for predicting wind direction and speed. Isotherms, lines representing equal temperature, enable the visualization of temperature distribution, aiding in forecasting temperature gradients and weather fronts.
Practical Applications of Isobars
Isobars are instrumental in meteorology for analyzing atmospheric pressure patterns, aiding in weather forecasting and identifying storm systems. In aviation, isobar charts help pilots determine wind flow and turbulence by depicting pressure variations at constant altitudes. Industrial processes such as chemical engineering use isobaric conditions to maintain pressure control during reactions, ensuring safety and efficiency.
Practical Applications of Isotherms
Isotherms, which represent constant temperature conditions on a graph, are critical in practical applications such as the design and analysis of phase changes in refrigeration systems and chemical reactors. Engineers use isotherms to optimize thermal processes, ensuring energy efficiency and stability in temperature-sensitive operations like cryogenics and polymer curing. These curves aid in predicting material behavior under varying pressure and volume while maintaining temperature, facilitating better control in industrial and environmental processes.
Conclusion: Isobar vs Isotherm
Isobar refers to a line or curve on a diagram representing constant pressure, while isotherm denotes a line of constant temperature. In thermodynamic processes, isobars highlight changes occurring under steady pressure conditions, whereas isotherms depict processes maintaining uniform temperature. Understanding the distinction between isobar and isotherm is critical for analyzing phase changes, heat transfer, and pressure-volume relationships in physical and chemical systems.
Isobar Infographic
 libterm.com
libterm.com