Nanocrystalline materials exhibit unique physical and chemical properties due to their extremely fine grain sizes, often less than 100 nanometers. These materials demonstrate enhanced strength, increased hardness, and improved catalytic activity compared to their coarse-grained counterparts. Explore the article to discover how nanocrystalline structures can impact your advanced material applications and innovations.
Table of Comparison
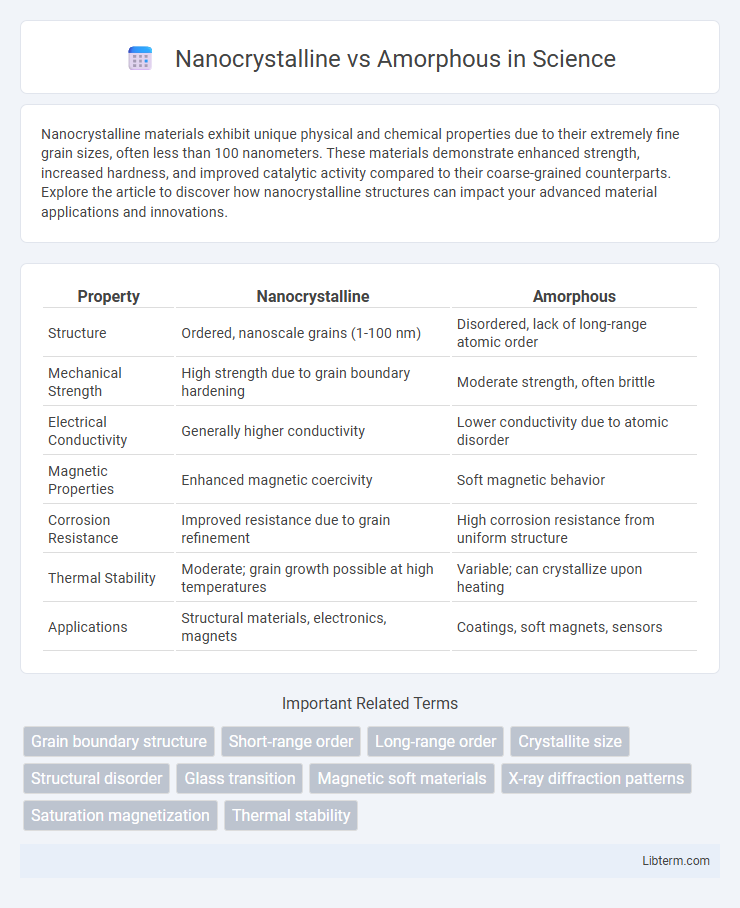
| Property | Nanocrystalline | Amorphous |
|---|---|---|
| Structure | Ordered, nanoscale grains (1-100 nm) | Disordered, lack of long-range atomic order |
| Mechanical Strength | High strength due to grain boundary hardening | Moderate strength, often brittle |
| Electrical Conductivity | Generally higher conductivity | Lower conductivity due to atomic disorder |
| Magnetic Properties | Enhanced magnetic coercivity | Soft magnetic behavior |
| Corrosion Resistance | Improved resistance due to grain refinement | High corrosion resistance from uniform structure |
| Thermal Stability | Moderate; grain growth possible at high temperatures | Variable; can crystallize upon heating |
| Applications | Structural materials, electronics, magnets | Coatings, soft magnets, sensors |
Introduction to Magnetic Materials
Nanocrystalline magnetic materials feature ultra-fine grains typically below 100 nm, resulting in enhanced magnetic properties such as high permeability and low coercivity due to reduced magnetocrystalline anisotropy. Amorphous magnetic materials lack long-range atomic order, which minimizes magnetic domain wall pinning and leads to soft magnetic behavior with excellent electrical resistivity. Both materials play crucial roles in advanced magnetic applications, including transformers, inductors, and magnetic sensors, owing to their distinctive microstructural and magnetic characteristics.
What are Nanocrystalline Materials?
Nanocrystalline materials consist of grains with sizes typically less than 100 nanometers, which significantly enhances their mechanical, thermal, and electrical properties compared to their coarse-grained counterparts. These materials exhibit a high density of grain boundaries, leading to increased strength, hardness, and improved diffusion rates. Nanocrystalline structures are widely used in applications such as catalysts, sensors, and advanced coatings due to their superior surface area and reactivity.
What are Amorphous Materials?
Amorphous materials lack a long-range ordered atomic structure, distinguishing them from crystalline and nanocrystalline materials. Their atoms are arranged randomly, resulting in unique properties such as isotropy and enhanced corrosion resistance. These materials are widely used in electronics, optics, and coatings due to their distinctive disordered atomic arrangement.
Structural Differences: Nanocrystalline vs Amorphous
Nanocrystalline materials consist of grains with sizes typically less than 100 nanometers, exhibiting a well-defined crystalline lattice structure, whereas amorphous materials lack long-range order, resulting in a disordered atomic arrangement. The presence of grain boundaries in nanocrystalline materials significantly influences their mechanical and electrical properties, contrasting with the homogeneous structure of amorphous phases. These structural differences directly impact properties such as strength, ductility, and thermal stability, making nanocrystalline materials distinct from their amorphous counterparts.
Magnetic Properties Comparison
Nanocrystalline materials exhibit enhanced magnetic properties such as higher saturation magnetization and lower coercivity compared to amorphous materials, due to their well-defined grain boundaries and uniform atomic structure. Amorphous alloys typically have isotropic magnetic behavior and higher electrical resistivity, reducing eddy current losses in transformers and magnetic cores. The magnetic softness of nanocrystalline alloys makes them ideal for high-frequency applications, while amorphous materials excel in environments requiring thermal stability and corrosion resistance.
Thermal Stability and Performance
Nanocrystalline materials exhibit superior thermal stability compared to amorphous materials due to their well-defined grain boundaries that impede atomic diffusion and phase transformation. The thermal performance of nanocrystalline structures is enhanced by their high melting points and resistance to softening under elevated temperatures, making them ideal for high-temperature applications. In contrast, amorphous materials often suffer from lower thermal stability, leading to structural relaxation and crystallization that degrade their mechanical and electrical properties over time.
Industrial Applications and Use Cases
Nanocrystalline materials exhibit enhanced mechanical strength, wear resistance, and magnetic properties, making them ideal for cutting tools, transformers, and high-performance coatings in industrial applications. Amorphous materials, characterized by their disordered atomic structure, offer superior corrosion resistance, soft magnetic behavior, and excellent thermal stability, widely used in electrical transformers, magnetic sensors, and thin-film coatings. Industries leverage nanocrystalline alloys for structural components requiring durability and amorphous metals for energy-efficient magnetic devices and advanced protective layers.
Advantages and Limitations
Nanocrystalline materials exhibit superior mechanical strength and enhanced electrical conductivity due to their ultra-fine grain structure, which promotes high grain boundary density and increased surface area. Amorphous materials, characterized by their disordered atomic arrangement, offer excellent corrosion resistance and soft magnetic properties, making them ideal for specific applications like transformers and magnetic sensors. However, nanocrystalline materials may suffer from thermal instability at elevated temperatures, while amorphous materials often have lower mechanical strength and limited ductility compared to their crystalline counterparts.
Recent Advances in Material Research
Recent advances in material research reveal significant distinctions between nanocrystalline and amorphous materials in terms of thermal stability and mechanical properties. Nanocrystalline materials exhibit enhanced strength and electrical conductivity due to their well-defined grain boundaries, while amorphous materials offer superior corrosion resistance and magnetic softness associated with their disordered atomic arrangement. Cutting-edge techniques such as high-resolution transmission electron microscopy (HR-TEM) and atom probe tomography (APT) have enabled detailed analysis of structural evolution, driving innovations in applications ranging from flexible electronics to high-performance magnetic devices.
Conclusion and Future Perspectives
Nanocrystalline materials exhibit superior mechanical strength and thermal stability compared to amorphous structures due to their ordered grain boundaries and reduced defect density. Emerging research highlights potential applications in electronics and energy storage, driven by their enhanced electrical conductivity and magnetic properties. Future developments will likely focus on scalable synthesis methods and tailored nanostructuring to optimize performance for industrial applications.
Nanocrystalline Infographic
 libterm.com
libterm.com