Oxidizing is a chemical process that involves the loss of electrons from a substance, often resulting in the combination with oxygen or the increase in oxidation state. This reaction plays a crucial role in various industrial applications, environmental processes, and biological systems, impacting everything from corrosion to cellular respiration. Discover how oxidizing affects your world and the mechanisms behind it in the rest of this article.
Table of Comparison
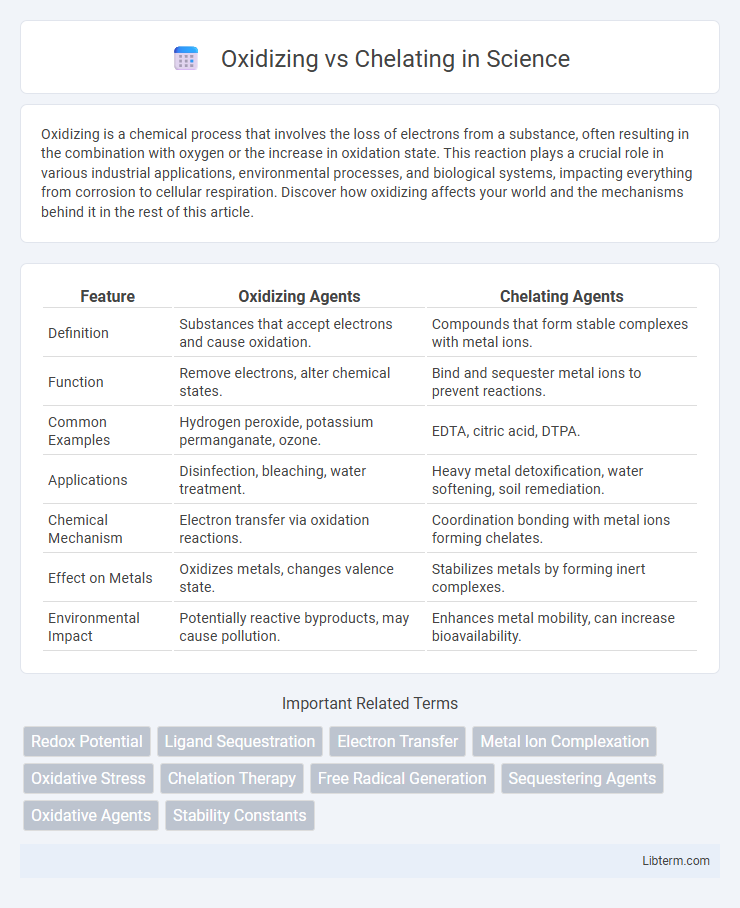
| Feature | Oxidizing Agents | Chelating Agents |
|---|---|---|
| Definition | Substances that accept electrons and cause oxidation. | Compounds that form stable complexes with metal ions. |
| Function | Remove electrons, alter chemical states. | Bind and sequester metal ions to prevent reactions. |
| Common Examples | Hydrogen peroxide, potassium permanganate, ozone. | EDTA, citric acid, DTPA. |
| Applications | Disinfection, bleaching, water treatment. | Heavy metal detoxification, water softening, soil remediation. |
| Chemical Mechanism | Electron transfer via oxidation reactions. | Coordination bonding with metal ions forming chelates. |
| Effect on Metals | Oxidizes metals, changes valence state. | Stabilizes metals by forming inert complexes. |
| Environmental Impact | Potentially reactive byproducts, may cause pollution. | Enhances metal mobility, can increase bioavailability. |
Introduction to Oxidizing and Chelating Agents
Oxidizing agents are substances that facilitate oxidation by accepting electrons, often used in chemical reactions to break down compounds or disinfect surfaces. Chelating agents bind to metal ions through multiple coordination sites, stabilizing metals and preventing unwanted reactions in industrial, pharmaceutical, or environmental applications. Understanding the distinct functions of oxidizing and chelating agents is essential for their effective application in processes like water treatment, catalysis, and metal recovery.
Defining Oxidizing Agents: Properties and Functions
Oxidizing agents are substances that accept electrons during a chemical reaction, facilitating oxidation by increasing the oxidation state of other molecules. Common oxidizing agents, such as hydrogen peroxide, potassium permanganate, and chlorine, exhibit strong electron affinity and release oxygen or reactive oxygen species to break down contaminants. These agents are widely utilized in disinfection, bleaching, and environmental remediation due to their ability to disrupt chemical bonds and degrade organic and inorganic pollutants efficiently.
Understanding Chelating Agents: Mechanisms and Applications
Chelating agents function by forming stable complexes with metal ions through multiple coordinate bonds, effectively neutralizing their reactivity and preventing oxidation reactions. Unlike oxidizing agents that transfer electrons to drive chemical changes, chelators stabilize metals such as iron, calcium, and magnesium, enhancing solubility and bioavailability or facilitating metal removal in detoxification processes. Applications of chelating agents span water treatment, pharmaceuticals, agriculture, and industrial processes, where precise control of metal ion activity is critical for optimizing performance and preventing corrosion or toxicity.
Key Differences Between Oxidizing and Chelating Agents
Oxidizing agents participate in redox reactions by accepting electrons, causing oxidation in other substances, whereas chelating agents form stable complexes with metal ions, preventing their participation in chemical reactions. Oxidizers like hydrogen peroxide and potassium permanganate are chiefly used to break down contaminants through electron transfer, while chelators such as EDTA or citric acid sequester metals to reduce toxicity or enhance solubility. The key difference lies in their chemical roles: oxidizing agents alter the oxidation state of molecules, while chelating agents selectively bind metal ions without changing their oxidation numbers.
Common Examples: Oxidizing vs Chelating Compounds
Common oxidizing compounds include hydrogen peroxide, potassium permanganate, and chlorine, which are widely used for their strong electron-accepting properties in disinfection and bleaching. Chelating compounds such as EDTA, DTPA, and citric acid bind metal ions selectively, preventing their catalytic activity and enhancing stability in industrial and environmental applications. The contrasting roles of these compounds highlight oxidizers' ability to drive redox reactions, while chelators stabilize metals to control reactivity and prevent unwanted side effects.
Industrial and Laboratory Uses: Side-by-Side Comparison
Oxidizing agents such as hydrogen peroxide and potassium permanganate are widely used in industrial processes like wastewater treatment and metal recovery for their ability to break down contaminants and facilitate oxidation reactions. Chelating agents like EDTA and citric acid are essential in laboratories and manufacturing for their capacity to bind metal ions, preventing precipitation and enhancing metal ion solubility during analytical and cleaning procedures. Both oxidizing and chelating agents improve process efficiency but serve distinct roles: oxidizers alter chemical states, while chelators stabilize metal ions for controlled reactions.
Safety Considerations and Handling Procedures
Oxidizing agents require strict safety measures due to their potential to cause fires or explosions when in contact with flammable materials, necessitating storage in cool, well-ventilated areas away from incompatible substances. Chelating agents, while generally less reactive, demand careful handling to avoid skin and eye irritation, and require the use of personal protective equipment such as gloves and goggles. Proper labeling, secure containers, and adherence to Material Safety Data Sheets (MSDS) guidelines are essential for both oxidizing and chelating chemicals to minimize health risks and environmental impact.
Environmental Impact: Oxidizing vs Chelating Agents
Oxidizing agents such as hydrogen peroxide and chlorine compounds can degrade organic pollutants but often generate harmful byproducts, posing risks to aquatic ecosystems and soil health. Chelating agents like EDTA and DTPA improve heavy metal mobility and bioavailability but persist in the environment, potentially causing metal remobilization and groundwater contamination. Choosing environmentally safer alternatives involves balancing effective pollutant removal with minimizing chemical persistence and ecological toxicity.
Choosing the Right Agent: Factors to Consider
Choosing the right agent between oxidizing and chelating depends on the specific chemical reaction and desired outcome. Oxidizing agents are preferred for processes requiring electron transfer to increase oxidation states, while chelating agents are ideal for stabilizing metal ions by forming coordination complexes. Consider factors such as target compounds, reaction conditions, toxicity, environmental impact, and compatibility with other substances when selecting the appropriate agent.
Conclusion: Optimizing Outcomes with Proper Agent Selection
Selecting the appropriate oxidizing or chelating agent significantly impacts the efficiency and safety of chemical processes in industries such as water treatment and pharmaceuticals. Oxidizing agents excel in breaking down contaminants through electron transfer, while chelating agents stabilize metal ions to prevent unwanted reactions and enhance bioavailability. Optimizing outcomes requires evaluating the specific chemical environment and desired reaction, ensuring agent compatibility to maximize effectiveness and minimize environmental impact.
Oxidizing Infographic
 libterm.com
libterm.com