Non-crystalline materials, often referred to as amorphous solids, lack the long-range order characteristic of crystalline structures, resulting in unique physical properties such as isotropy and variable optical transparency. These materials are widely used in applications ranging from glass manufacturing to advanced electronics due to their flexibility and adaptability. Discover how non-crystalline materials impact modern technology and your daily life by exploring the rest of this article.
Table of Comparison
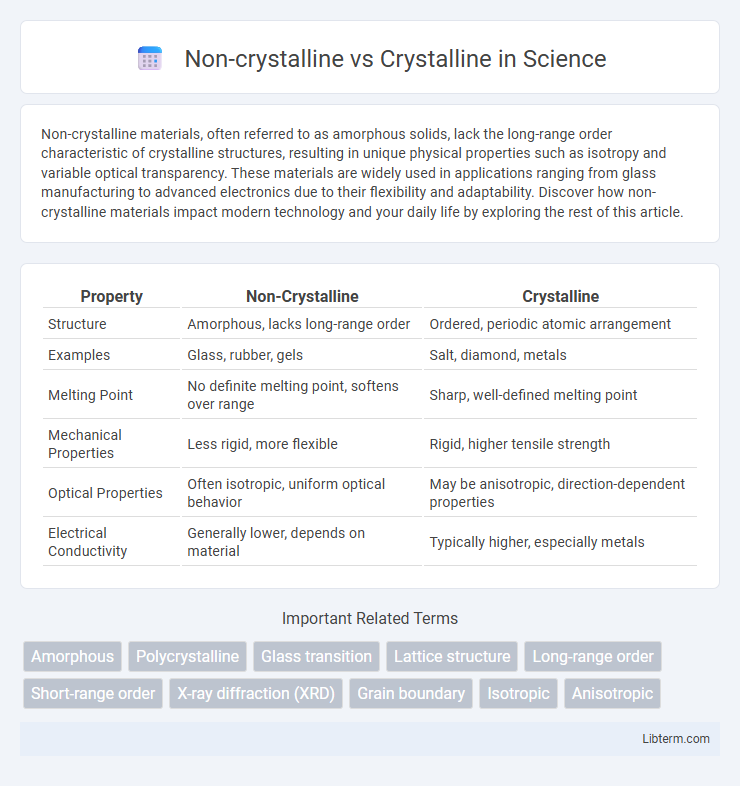
| Property | Non-Crystalline | Crystalline |
|---|---|---|
| Structure | Amorphous, lacks long-range order | Ordered, periodic atomic arrangement |
| Examples | Glass, rubber, gels | Salt, diamond, metals |
| Melting Point | No definite melting point, softens over range | Sharp, well-defined melting point |
| Mechanical Properties | Less rigid, more flexible | Rigid, higher tensile strength |
| Optical Properties | Often isotropic, uniform optical behavior | May be anisotropic, direction-dependent properties |
| Electrical Conductivity | Generally lower, depends on material | Typically higher, especially metals |
Introduction to Non-crystalline and Crystalline Materials
Non-crystalline materials, also known as amorphous solids, lack a long-range ordered atomic structure, resulting in isotropic properties and varied mechanical behavior compared to crystalline materials. Crystalline materials exhibit a well-defined, repeating atomic lattice structure, which imparts anisotropic properties and predictable physical characteristics such as melting points and electrical conductivity. Understanding these structural differences is crucial for applications in electronics, optics, and materials engineering.
Defining Crystalline Structures
Crystalline structures are characterized by an ordered, repeating arrangement of atoms or molecules forming a lattice, which imparts distinct physical properties such as defined melting points and anisotropy. Non-crystalline materials, also known as amorphous solids, lack this long-range periodic atomic order, resulting in isotropic properties and a gradual transition between solid and liquid phases. Understanding crystalline structures involves analyzing unit cells, lattice parameters, and symmetry, which are essential for determining material behavior and applications in fields like metallurgy and semiconductor technology.
Understanding Non-crystalline (Amorphous) Structures
Non-crystalline or amorphous structures lack the long-range order that defines crystalline materials, leading to distinct physical properties such as isotropy and varying degrees of transparency. Atomic arrangement in amorphous solids is random, resulting in variations in density and mechanical behavior compared to the uniform lattice structure of crystalline solids. Understanding these differences is crucial in materials science for applications involving glasses, polymers, and thin films where non-crystalline characteristics influence performance and durability.
Atomic Arrangement and Structural Order
Non-crystalline materials, also known as amorphous solids, exhibit a random atomic arrangement lacking long-range structural order, which contrasts with the periodic and highly ordered atomic lattice found in crystalline materials. The absence of repeating units in non-crystalline substances leads to isotropic properties, whereas crystalline solids display anisotropy due to their well-defined atomic planes and symmetry. Structural order in crystalline materials results in sharp diffraction patterns, while non-crystalline materials produce broad, diffuse patterns reflective of their short-range order only.
Physical Properties: Hardness, Density, and Strength
Non-crystalline materials, such as glasses and amorphous solids, typically exhibit lower hardness and strength compared to crystalline counterparts due to their disordered atomic structure, which lacks long-range order and slip planes that facilitate plastic deformation. Crystalline materials, including metals and ceramics, possess higher density and mechanical strength because their atoms are arranged in a regular, repeating lattice that enhances atomic packing efficiency and resistance to deformation. The variation in physical properties between non-crystalline and crystalline solids significantly influences their applications, with crystalline materials favored for high-stress environments requiring superior hardness and strength.
Optical and Electrical Characteristics
Non-crystalline materials, such as amorphous silicon, exhibit lower optical transparency and higher light scattering due to the irregular atomic arrangement, resulting in reduced optical clarity compared to crystalline materials. Crystalline substances, with their well-ordered atomic structures, demonstrate superior optical properties including higher refractive indices and lower absorption losses, which are critical for efficient optoelectronic devices. Electron mobility and electrical conductivity are significantly higher in crystalline materials owing to fewer defects and grain boundaries, whereas non-crystalline materials typically show lower electrical performance but offer advantages like flexibility and ease of fabrication.
Formation and Processing Methods
Non-crystalline materials, also known as amorphous solids, form through rapid cooling or quenching, preventing atoms from arranging into a regular lattice, as seen in materials like glass and certain polymers. Crystalline materials develop through slow cooling or controlled solidification, allowing atoms to organize into highly ordered, repeating patterns typical of metals, salts, and minerals. Processing methods for non-crystalline materials often involve techniques such as melt spinning and vapor deposition, while crystalline materials undergo processes like annealing and controlled crystallization to optimize grain structure and mechanical properties.
Applications and Industrial Uses
Crystalline materials, characterized by their ordered atomic structure, are extensively used in electronics and optics for components like semiconductors, solar cells, and laser devices due to their predictable electrical and thermal properties. Non-crystalline materials, or amorphous solids, find applications in flexible displays, thin-film solar panels, and scratch-resistant coatings because of their isotropic properties and ease of manufacturing. Industrially, crystalline substances dominate in mechanical and structural applications requiring high strength and thermal stability, while non-crystalline materials excel in uses demanding uniformity and malleability, such as glass manufacturing and polymer-based products.
Advantages and Limitations of Each Type
Non-crystalline materials, such as glass and amorphous metals, offer advantages like isotropic properties and ease of fabrication but suffer from lower mechanical strength and thermal stability compared to crystalline counterparts. Crystalline materials exhibit well-ordered atomic structures that contribute to superior strength, thermal conductivity, and electronic properties, yet they often face limitations including anisotropy and complex manufacturing processes. Selecting between non-crystalline and crystalline materials depends on application-specific requirements for mechanical durability, thermal resistance, and manufacturing constraints.
Future Trends in Material Science
Future trends in material science highlight the increasing exploration of non-crystalline materials such as metallic glasses for their superior strength and corrosion resistance compared to traditional crystalline counterparts. Advances in nanotechnology and machine learning-driven material design accelerate the discovery of amorphous solids with tailored properties for aerospace, electronics, and biomedical applications. Research efforts prioritize optimizing the thermal stability and mechanical performance of non-crystalline materials to overcome current limitations and expand their industrial viability.
Non-crystalline Infographic
 libterm.com
libterm.com