Proliferative refers to the rapid and excessive growth or multiplication of cells, tissues, or organisms, often associated with biological processes such as wound healing or pathological conditions like cancer. Understanding proliferative mechanisms is crucial for developing targeted treatments and managing diseases characterized by abnormal cell proliferation. Explore this article to deepen your knowledge of proliferative processes and their implications for health and medicine.
Table of Comparison
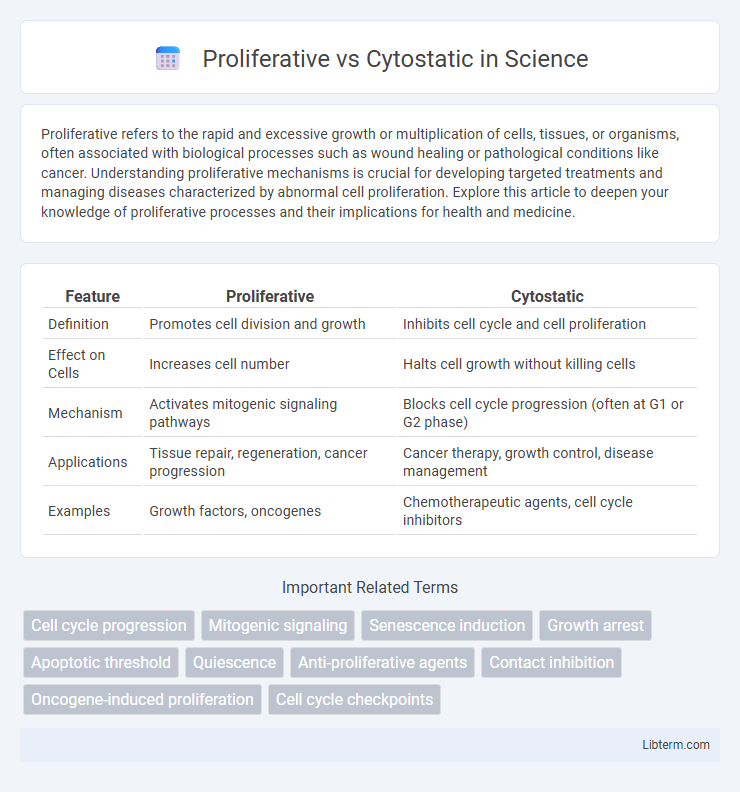
| Feature | Proliferative | Cytostatic |
|---|---|---|
| Definition | Promotes cell division and growth | Inhibits cell cycle and cell proliferation |
| Effect on Cells | Increases cell number | Halts cell growth without killing cells |
| Mechanism | Activates mitogenic signaling pathways | Blocks cell cycle progression (often at G1 or G2 phase) |
| Applications | Tissue repair, regeneration, cancer progression | Cancer therapy, growth control, disease management |
| Examples | Growth factors, oncogenes | Chemotherapeutic agents, cell cycle inhibitors |
Introduction to Proliferative and Cytostatic Mechanisms
Proliferative mechanisms involve cellular processes that drive cell division and growth, often regulated by signaling pathways such as cyclins and growth factors like epidermal growth factor (EGF). Cytostatic mechanisms inhibit cell proliferation by inducing cell cycle arrest through pathways involving tumor suppressors like p53 and CDK inhibitors such as p21 and p27. Understanding the balance between proliferative signals and cytostatic checkpoints is essential for targeting diseases characterized by uncontrolled cell growth, including cancer.
Defining Proliferative Responses in Cells
Proliferative responses in cells are characterized by increased cell division and growth, driven by signaling pathways that promote cell cycle progression and DNA replication. These responses contrast with cytostatic effects, where cell proliferation is halted without inducing cell death. Key molecular markers such as cyclins, CDKs, and growth factors help define and distinguish proliferative activity from cytostatic states in cellular biology.
Understanding Cytostatic Effects and Actions
Cytostatic effects inhibit cell proliferation by arresting the cell cycle, preventing cells from dividing without inducing cell death, contrasting with proliferative processes that promote cell multiplication. Mechanisms underlying cytostatic actions often involve modulation of cyclin-dependent kinases (CDKs), tumor suppressor pathways like p53, and interruption of signaling cascades crucial for cell cycle progression. Understanding these molecular targets enables the development of therapeutic agents aimed at controlling pathological cell growth in cancer and hyperproliferative diseases.
Key Molecular Pathways: Proliferative vs Cytostatic
Proliferative pathways primarily involve the activation of growth factor receptors such as EGFR and downstream signaling cascades like the MAPK/ERK and PI3K/AKT pathways, driving cell cycle progression and mitosis. In contrast, cytostatic effects commonly engage tumor suppressor pathways including p53 and Rb, leading to cell cycle arrest through cyclin-dependent kinase inhibitors like p21 and p27. The balance between these pathways determines cellular outcomes, influencing tissue growth, cancer progression, and responses to therapeutic agents targeting proliferative signaling.
Cellular Outcomes: Growth versus Growth Arrest
Proliferative cellular outcomes promote continuous cell division and expansion, driving tissue growth and regeneration. Cytostatic effects induce growth arrest, halting the cell cycle without triggering cell death, often as a response to stress or therapeutic intervention. Understanding the balance between proliferative signals and cytostatic mechanisms is crucial for targeting diseases characterized by abnormal cell growth, such as cancer.
Clinical Implications in Cancer Therapy
Proliferative cancer therapies target rapidly dividing tumor cells to reduce tumor burden but may cause resistance due to selective pressure on aggressive clones. Cytostatic agents halt cancer cell growth and division without immediate cell death, potentially offering prolonged disease stabilization with fewer side effects. Understanding the balance between proliferative and cytostatic effects informs personalized treatment strategies, optimizing efficacy while minimizing toxicity in cancer therapy.
Advantages and Limitations of Proliferative Strategies
Proliferative strategies promote cell growth and regeneration, offering advantages in tissue repair and recovery by accelerating cellular functions and enhancing regenerative capacity. However, these approaches carry limitations such as increased risk of uncontrolled cell proliferation, potential tumorigenesis, and difficulty in precisely regulating the growth rate. Effective application requires balancing regenerative benefits with the risk of adverse effects like hyperplasia or malignancy.
Strengths and Challenges of Cytostatic Approaches
Cytostatic approaches primarily inhibit cancer cell proliferation by arresting the cell cycle, which helps to control tumor growth without inducing extensive cell death, reducing collateral damage to healthy tissues. Their strength lies in minimizing toxicity and resistance development compared to cytotoxic therapies, making them suitable for long-term management of chronic cancers. Challenges include limited effectiveness against rapidly dividing cells, potential tumor dormancy leading to relapse, and the need for precise molecular targeting to overcome adaptive cellular mechanisms.
Biomarkers for Assessing Proliferative and Cytostatic States
Biomarkers such as Ki-67 and PCNA are essential for assessing proliferative states, indicating active cell division and growth. Cytostatic conditions are often evaluated using markers like p21 and p27, which signal cell cycle arrest and inhibition of proliferation. Accurate measurement of these biomarkers facilitates the distinction between proliferative and cytostatic cellular environments, crucial for cancer diagnostics and therapeutic strategies.
Future Directions in Targeting Proliferative and Cytostatic Pathways
Emerging therapies targeting proliferative pathways focus on inhibiting key cell cycle regulators such as cyclin-dependent kinases (CDKs) and growth factor signaling to halt tumor growth. Future directions emphasize combination strategies that integrate cytostatic agents, which induce senescence or metabolic dormancy, to enhance treatment durability and prevent resistance. Advances in single-cell sequencing and biomarker identification will enable precise modulation of proliferative and cytostatic states, optimizing personalized cancer therapeutics.
Proliferative Infographic
 libterm.com
libterm.com