Distillation is a critical process used to separate mixtures based on differences in boiling points, widely applied in industries such as petrochemical refining, alcohol production, and water purification. It ensures the efficient extraction of valuable components, improving product quality and safety. Discover how distillation can optimize your processes and why it remains indispensable by reading the full article.
Table of Comparison
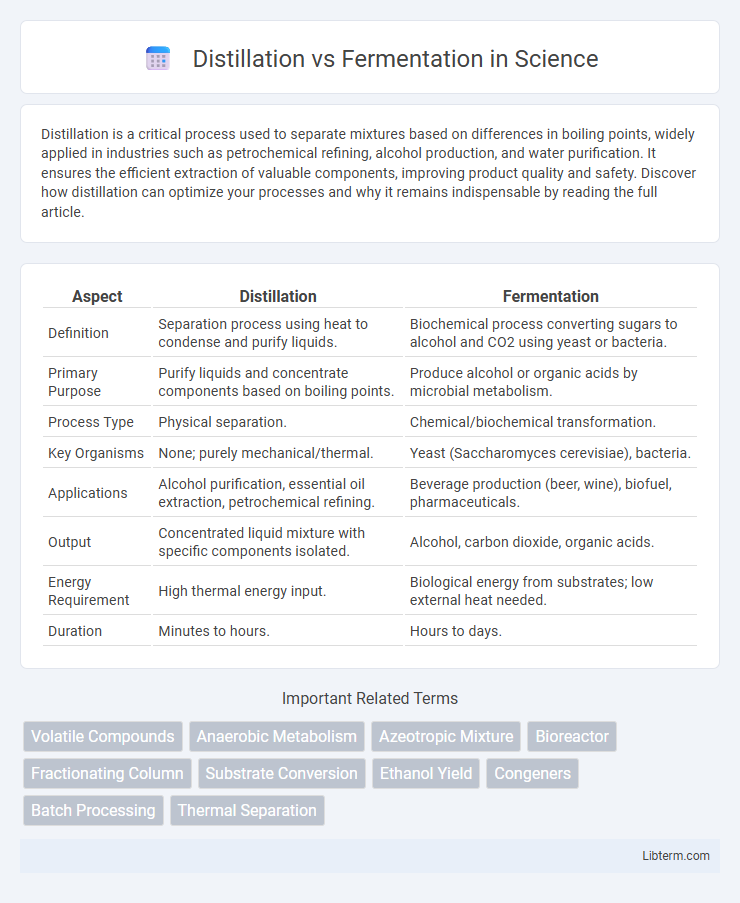
| Aspect | Distillation | Fermentation |
|---|---|---|
| Definition | Separation process using heat to condense and purify liquids. | Biochemical process converting sugars to alcohol and CO2 using yeast or bacteria. |
| Primary Purpose | Purify liquids and concentrate components based on boiling points. | Produce alcohol or organic acids by microbial metabolism. |
| Process Type | Physical separation. | Chemical/biochemical transformation. |
| Key Organisms | None; purely mechanical/thermal. | Yeast (Saccharomyces cerevisiae), bacteria. |
| Applications | Alcohol purification, essential oil extraction, petrochemical refining. | Beverage production (beer, wine), biofuel, pharmaceuticals. |
| Output | Concentrated liquid mixture with specific components isolated. | Alcohol, carbon dioxide, organic acids. |
| Energy Requirement | High thermal energy input. | Biological energy from substrates; low external heat needed. |
| Duration | Minutes to hours. | Hours to days. |
Understanding Distillation and Fermentation
Distillation is a process that separates mixtures based on differences in boiling points, primarily used to purify liquids or extract essential oils and alcohol. Fermentation is a biochemical process where microorganisms like yeast convert sugars into alcohol or acids under anaerobic conditions, playing a crucial role in producing alcoholic beverages and biofuels. Understanding distillation involves grasping phase changes and vapor-liquid equilibrium, while fermentation requires knowledge of metabolic pathways and enzymatic activity in microbial organisms.
Key Differences Between Distillation and Fermentation
Distillation is a physical separation process that concentrates alcohol by heating a fermented liquid to separate ethanol from other components based on boiling points, while fermentation is a biochemical process where yeast converts sugars into alcohol and carbon dioxide. Distillation typically follows fermentation to increase alcohol content, producing spirits like whiskey and vodka, whereas fermentation alone creates products such as beer, wine, and cider with lower alcohol levels. The key difference lies in fermentation generating alcohol through microbial activity, whereas distillation refines and intensifies that alcohol content through evaporation and condensation.
The Science Behind Distillation
Distillation relies on the principle of boiling point differences to separate components in a liquid mixture, allowing for the concentration of alcohol by heating fermented liquid and condensing the vapor. The process exploits the volatility of ethanol, which boils at 78.37degC, lower than water's 100degC, enabling ethanol to vaporize first for collection. Precise temperature control and repeated distillation cycles enhance purity and alcohol content, distinct from fermentation where yeast metabolizes sugars into alcohol and carbon dioxide.
The Principles of Fermentation
Fermentation is a biochemical process that converts sugars into alcohol and carbon dioxide using yeast or bacteria under anaerobic conditions. This biological reaction primarily relies on enzymes like zymase to break down glucose molecules, producing ethanol and energy. Unlike distillation, which separates alcohol from a liquid mixture by heating and condensation, fermentation itself generates alcohol through metabolic activity.
Processes Involved in Distillation
Distillation involves heating a liquid mixture to create vapor and then cooling the vapor to collect the separate components based on different boiling points, commonly used to purify alcohol or essential oils. This process includes stages such as heating, vaporization, condensation, and collection within distillation columns or pot stills. Unlike fermentation, which relies on biochemical conversion of sugars by yeast to produce alcohol, distillation physically separates compounds to increase alcohol concentration and purity.
Steps of the Fermentation Process
The fermentation process begins with substrate preparation, where sugars from sources like grains or fruits are extracted and sterilized to prevent contamination. Yeast or bacteria are then introduced to convert these sugars into ethanol and carbon dioxide through anaerobic metabolism. Finally, the fermented mixture is clarified and conditioned before distillation, where ethanol is purified by heating and condensation.
Common Applications: Distillation vs Fermentation
Distillation is commonly applied in the production of spirits such as whiskey, vodka, and rum by separating alcohol from fermented liquids to increase purity and alcohol concentration. Fermentation is primarily used in brewing beer, producing wine, and creating biofuels by converting sugars into alcohol and carbon dioxide through yeast or bacteria activity. Both processes play essential roles in the beverage industry, with fermentation initiating alcohol production and distillation refining the final product for higher strength and flavor profiles.
Advantages and Disadvantages of Each Method
Distillation offers the advantage of producing a high-purity alcohol concentration, ideal for spirits, yet it requires significant energy input and specialized equipment. Fermentation is cost-effective and natural, utilizing yeast to convert sugars into alcohol, but it results in lower alcohol content and longer processing times. Choosing between distillation and fermentation depends on the desired alcohol strength, production scale, and resource availability.
Safety Considerations in Distillation and Fermentation
Distillation involves heating liquids to separate components based on boiling points, presenting fire and explosion risks due to flammable vapors and high temperatures, necessitating proper ventilation and explosion-proof equipment. Fermentation, while generally safer, requires careful monitoring of CO2 buildup in closed containers to prevent pressure hazards and contamination control to avoid harmful microbial growth. Both processes demand rigorous sanitation and adherence to safety protocols to protect operators and ensure product integrity.
Choosing the Right Technique for Your Needs
Choosing between distillation and fermentation depends on the desired end product and application. Fermentation is ideal for producing alcohol content in beverages like beer and wine through natural yeast activity converting sugars into ethanol. Distillation further refines these alcoholic products by separating components based on boiling points to increase purity and concentration, essential for spirits such as whiskey or vodka.
Distillation Infographic
 libterm.com
libterm.com