Pentose sugars are five-carbon monosaccharides essential in biological processes such as nucleic acid formation and energy metabolism. They play a crucial role in structures like ribose and deoxyribose, which are key components of RNA and DNA. Discover how pentoses impact your cellular functions and the broader implications of their molecular structure in the following article.
Table of Comparison
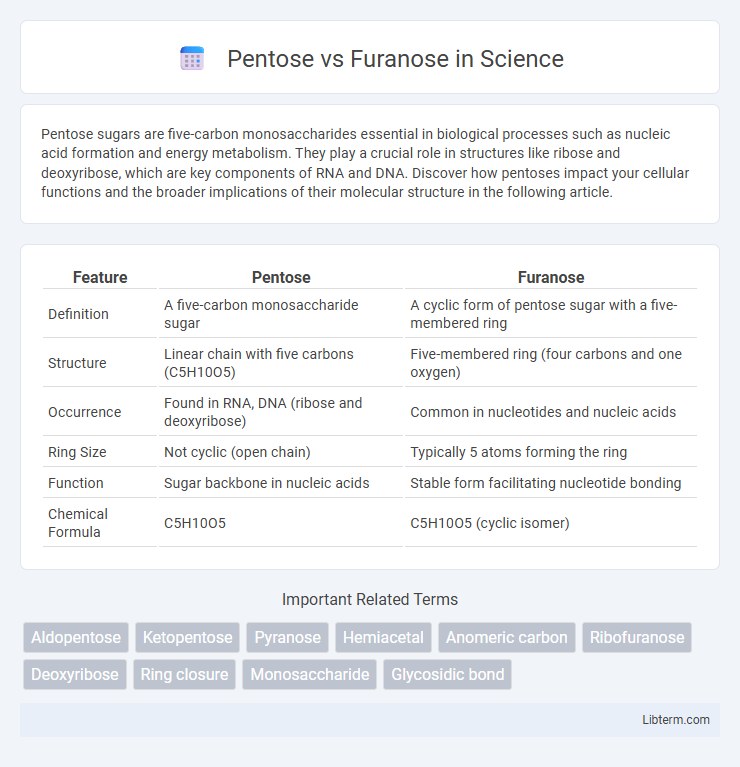
| Feature | Pentose | Furanose |
|---|---|---|
| Definition | A five-carbon monosaccharide sugar | A cyclic form of pentose sugar with a five-membered ring |
| Structure | Linear chain with five carbons (C5H10O5) | Five-membered ring (four carbons and one oxygen) |
| Occurrence | Found in RNA, DNA (ribose and deoxyribose) | Common in nucleotides and nucleic acids |
| Ring Size | Not cyclic (open chain) | Typically 5 atoms forming the ring |
| Function | Sugar backbone in nucleic acids | Stable form facilitating nucleotide bonding |
| Chemical Formula | C5H10O5 | C5H10O5 (cyclic isomer) |
Introduction to Pentose and Furanose
Pentose is a five-carbon sugar molecule commonly found in nucleotides and nucleic acids, playing a crucial role in RNA and DNA structure. Furanose refers to the cyclic form of sugars, including pentoses, characterized by a five-membered ring consisting of four carbons and one oxygen atom. The conversion of linear pentoses into furanose rings is essential for the stability and biological function of nucleic acids.
Definition of Pentose Sugars
Pentose sugars are monosaccharides that contain five carbon atoms, typically structured as either linear or cyclic forms. Furanose refers specifically to the cyclic form of pentose sugars that resembles a five-membered ring with four carbon atoms and one oxygen atom. These furanose rings are important structural components in nucleotides such as ribose in RNA and deoxyribose in DNA.
Understanding Furanose Structures
Furanose structures are five-membered ring forms of pentose sugars, including four carbon atoms and one oxygen atom, which create a cyclic hemiacetal or hemiketal. The ring formation in furanoses influences sugar reactivity and conformation, commonly found in nucleotides like ribose in RNA. Understanding furanose conformations, such as envelope or twist forms, is essential for studying carbohydrate chemistry and biochemical interactions.
Chemical Composition: Pentose vs Furanose
Pentose is a five-carbon monosaccharide with the general formula C5H10O5, whereas furanose refers to the cyclic form of sugars that resemble a five-membered ring consisting of four carbons and one oxygen atom. In chemical composition, pentoses include linear forms like ribose and xylose, while their furanose forms result from intramolecular cyclization creating a ring structure. The transition from pentose to furanose involves the formation of a hemiacetal linkage between the aldehyde or ketone group and a hydroxyl group on the sugar molecule.
Structural Differences Between Pentose and Furanose
Pentose refers to a five-carbon sugar molecule, which can exist in linear or ring forms, while furanose specifically describes the five-membered ring structure that includes four carbons and one oxygen atom. The key structural difference lies in the ring formation: pentose sugars form furanose rings when the aldehyde or ketone group reacts with the hydroxyl group on the fourth carbon, creating a cyclic hemiacetal or hemiketal. This ring closure changes the spatial configuration and properties of the sugar compared to its open-chain pentose form.
Biological Roles of Pentose and Furanose
Pentoses such as ribose and deoxyribose are crucial for forming nucleotides, the building blocks of RNA and DNA, which store and transmit genetic information in all living organisms. Furanose forms, the cyclic five-membered ring structures of these sugars, stabilize nucleic acid structures by enabling proper base pairing and backbone flexibility. The structural role of furanose in nucleotides directly influences the biochemical processes of transcription, replication, and energy metabolism through molecules like ATP.
Pentose in Nucleic Acids
Pentose sugars in nucleic acids primarily exist as five-carbon monosaccharides, crucial for forming the sugar-phosphate backbone of DNA and RNA. Ribose in RNA and deoxyribose in DNA adopt a furanose ring structure, a five-membered ring containing four carbons and one oxygen, enhancing molecular stability and facilitating nucleotide bonding. The pentose sugar's 2' hydroxyl group in ribose contributes to RNA's reactivity, whereas its absence in deoxyribose stabilizes DNA, making pentose configuration vital for nucleic acid function and structure.
Furanose in Carbohydrate Chemistry
Furanose refers to a five-membered ring structure in carbohydrates, characterized by four carbon atoms and one oxygen atom forming the ring, typically found in the cyclic forms of pentoses like ribose and fructose. This ring formation is crucial in nucleic acids, where ribose furanose serves as the sugar backbone of RNA, influencing molecular stability and enzymatic interactions. Understanding the furanose ring's stereochemistry is vital for studying carbohydrate reactivity and glycosidic bond formation in biochemical pathways.
Importance in Biochemistry and Health
Pentose sugars, specifically ribose and deoxyribose, form the backbone of nucleotides and nucleic acids, crucial for genetic information storage and transfer in biochemistry. Furanose refers to the five-membered ring form of these sugars, which stabilizes the structural conformation of RNA and DNA molecules, influencing their biological functions. Proper functioning of pentose and furanose forms is essential for cellular metabolism, energy production, and overall health, with imbalances linked to metabolic disorders and diseases like diabetes.
Key Differences and Summary
Pentose sugars are five-carbon monosaccharides that exist in both linear and cyclic forms, while furanose refers specifically to the five-membered ring form of these sugars, resembling a furan structure. The key difference lies in structure: pentoses can cyclize into furanose rings, where the ring includes four carbons and one oxygen, or into pyranose rings containing six atoms; furanose forms are less stable yet crucial in nucleotides like ribose in RNA. In summary, pentose defines the sugar's carbon count, and furanose describes its specific cyclic configuration, with important implications for biochemical roles and molecular stability.
Pentose Infographic
 libterm.com
libterm.com