Organophilic substances exhibit a strong affinity for organic compounds, making them essential in various chemical and industrial processes. Their compatibility with organic solvents enhances the efficiency of applications such as coatings, adhesives, and detergents. Discover how understanding organophilic properties can improve your use of these materials by reading the full article.
Table of Comparison
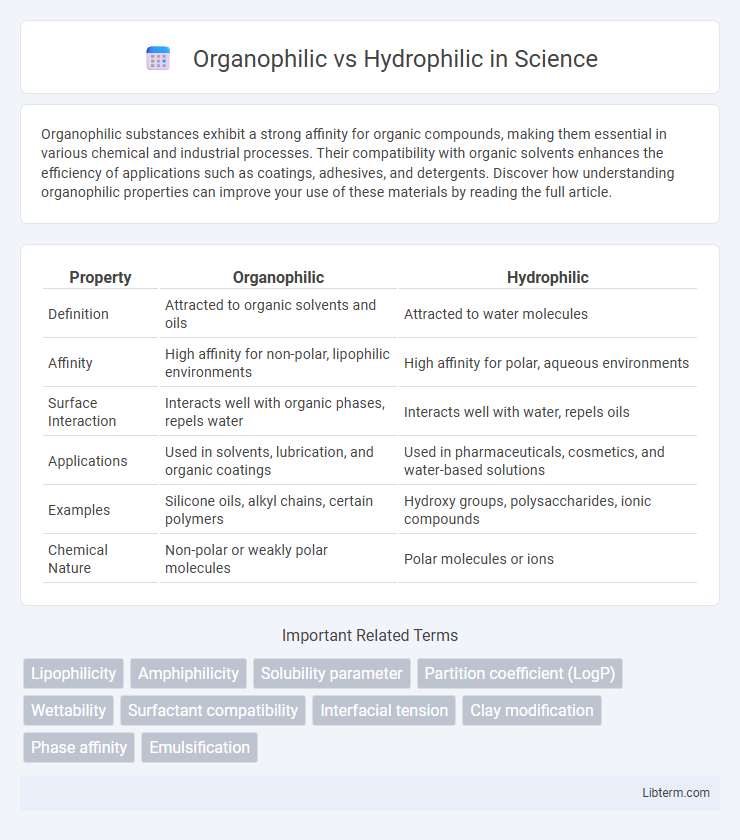
| Property | Organophilic | Hydrophilic |
|---|---|---|
| Definition | Attracted to organic solvents and oils | Attracted to water molecules |
| Affinity | High affinity for non-polar, lipophilic environments | High affinity for polar, aqueous environments |
| Surface Interaction | Interacts well with organic phases, repels water | Interacts well with water, repels oils |
| Applications | Used in solvents, lubrication, and organic coatings | Used in pharmaceuticals, cosmetics, and water-based solutions |
| Examples | Silicone oils, alkyl chains, certain polymers | Hydroxy groups, polysaccharides, ionic compounds |
| Chemical Nature | Non-polar or weakly polar molecules | Polar molecules or ions |
Understanding Organophilic and Hydrophilic Properties
Organophilic substances exhibit affinity for organic solvents and nonpolar materials due to their lipophilic molecular structure, enhancing compatibility with oils and hydrocarbons. Hydrophilic materials possess polar groups that attract and bond with water molecules, promoting solubility and dispersion in aqueous environments. Understanding the differential interactions of organophilic and hydrophilic properties is crucial in applications such as coating formulations, surfactants, and polymer composites where solvent compatibility and moisture resistance are key.
Chemical Definitions and Key Differences
Organophilic substances exhibit affinity for organic solvents and non-polar compounds due to their hydrophobic molecular structures, whereas hydrophilic substances have polar groups that enable strong interactions with water molecules. Chemically, organophilic materials contain non-polar hydrocarbon chains that facilitate solubility in oils, while hydrophilic materials possess polar functional groups like hydroxyl, carboxyl, or amine groups, enhancing solubility in aqueous environments. The key difference lies in their molecular polarity and solubility behavior, determining their compatibility with either organic or aqueous phases.
Molecular Structure and Interaction
Organophilic molecules contain nonpolar hydrocarbon chains that interact favorably with organic solvents through van der Waals forces, while hydrophilic molecules possess polar functional groups such as hydroxyl, carboxyl, or amino groups that form hydrogen bonds and ionic interactions with water. The molecular structure of organophilic substances promotes solubility in lipophilic environments by minimizing polarity, whereas hydrophilic compounds have polar or charged regions that enhance affinity for aqueous environments. These intrinsic differences in molecular polarity and functional group composition dictate the preferential interaction of organophilic and hydrophilic molecules with their respective solvents.
Common Organophilic Materials and Examples
Common organophilic materials include organoclays modified with quaternary ammonium salts, which enhance compatibility with organic solvents and oils by increasing hydrophobic interactions. Examples of organophilic substances are montmorillonite clay treated with alkylammonium ions and silanes that improve dispersion in polymer matrices. These materials are widely used in applications such as oil spill remediation, polymer nanocomposites, and cosmetics due to their affinity for organic phases and improved interfacial properties.
Typical Hydrophilic Substances and Applications
Typical hydrophilic substances include alcohols, sugars, and salts, which readily dissolve in water due to their polar molecular structure. These compounds are widely used in pharmaceuticals for drug formulation, in cosmetics for moisturizing products, and in agriculture for water-soluble fertilizers. Hydrophilic materials enable enhanced interaction with aqueous environments, promoting better solubility, bioavailability, and moisture retention in various industrial and biomedical applications.
Industrial Uses of Organophilic Compounds
Organophilic compounds exhibit excellent compatibility with organic solvents and oils, making them essential in industries such as coatings, adhesives, and lubricants where water repellency and oil affinity are required. Their ability to enhance the dispersion of pigments and fillers in non-aqueous systems improves product performance and durability in applications like paints and sealants. Organophilic clays and surfactants are widely utilized for their rheological control and stabilization properties in drilling fluids, cosmetics, and polymer processing.
Hydrophilic Compounds in Everyday Life
Hydrophilic compounds, characterized by their affinity for water due to polar groups such as hydroxyl (-OH) and amino (-NH2), play a crucial role in various everyday applications including skincare products, where they promote moisture retention and absorption. These compounds are essential in pharmaceuticals for enhanced drug solubility and bioavailability, as well as in food industries for texture and preservation through hydration. Common hydrophilic substances like glycerol, polyethylene glycol, and certain proteins demonstrate excellent water compatibility, underpinning their widespread use across hygiene, health, and nutrition sectors.
Blending and Compatibility: Organophilic vs Hydrophilic Substances
Organophilic substances exhibit strong compatibility with organic solvents and polymers, facilitating homogeneous blending in non-polar matrices, whereas hydrophilic substances show affinity for water-based systems, promoting dispersion in polar environments. The molecular interactions driving organophilic compatibility often involve van der Waals forces and hydrophobic interactions, while hydrophilic compatibility relies on hydrogen bonding and polarity. Optimizing blends requires selecting organophilic or hydrophilic agents based on solvent polarity and desired material properties to ensure phase stability and effective dispersion.
Impact on Environmental Behavior and Safety
Organophilic substances exhibit strong affinity for organic compounds, leading to accumulation in nonpolar environments and persistence in soils and sediments, which can increase toxicity and bioaccumulation risks for aquatic organisms. Hydrophilic substances readily dissolve in water, facilitating dispersion and biodegradation but also potentially increasing mobility in aquatic systems and groundwater contamination. Understanding the balance between organophilic and hydrophilic properties is crucial for assessing environmental fate, human exposure risks, and designing safer chemical formulations.
Choosing the Right Material: Organophilic or Hydrophilic
Choosing the right material between organophilic and hydrophilic types depends primarily on the target fluid and application environment. Organophilic materials excel in oil-based systems due to their affinity for non-polar compounds, making them ideal for oil spill remediation and organic solvent separation. Hydrophilic materials, in contrast, attract and absorb water, proving essential for applications like wastewater treatment, moisture control, and aqueous solution filtration.
Organophilic Infographic
 libterm.com
libterm.com