Tonic water is a carbonated beverage infused with quinine, giving it a distinctive bitter flavor that pairs well with various spirits, especially gin. Its unique taste and subtle bitterness make it a popular mixer for cocktails, offering a refreshing and slightly tangy experience. Explore the rest of the article to discover how tonic water can enhance your drink-making skills and refresh your palate.
Table of Comparison
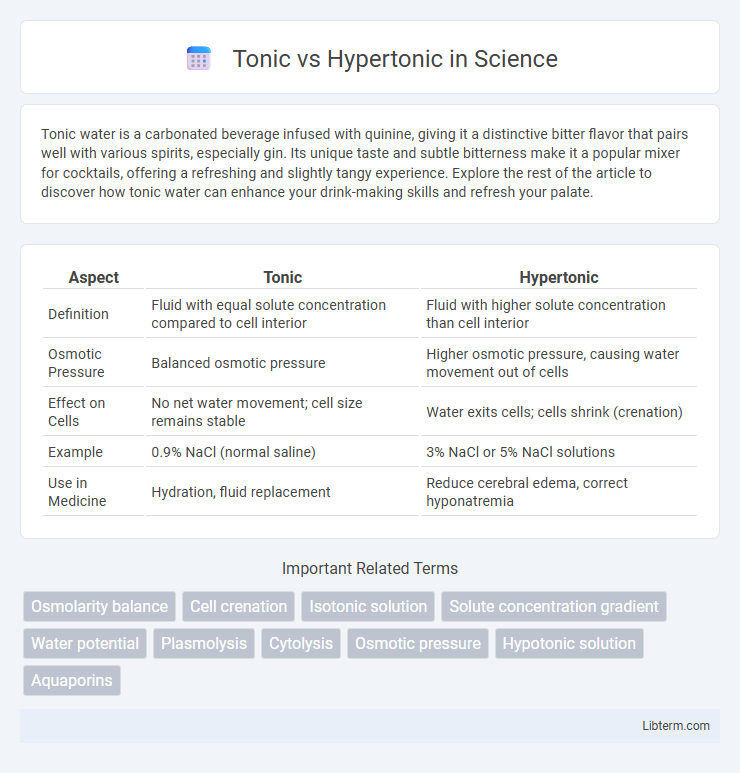
| Aspect | Tonic | Hypertonic |
|---|---|---|
| Definition | Fluid with equal solute concentration compared to cell interior | Fluid with higher solute concentration than cell interior |
| Osmotic Pressure | Balanced osmotic pressure | Higher osmotic pressure, causing water movement out of cells |
| Effect on Cells | No net water movement; cell size remains stable | Water exits cells; cells shrink (crenation) |
| Example | 0.9% NaCl (normal saline) | 3% NaCl or 5% NaCl solutions |
| Use in Medicine | Hydration, fluid replacement | Reduce cerebral edema, correct hyponatremia |
Understanding Tonic and Hypertonic Solutions
Tonic and hypertonic solutions differ primarily in solute concentration relative to a cell's cytoplasm, impacting osmotic pressure and water movement across the cell membrane. Tonic solutions, whether isotonic or hypotonic, maintain or reduce osmotic pressure, promoting balanced or inward water flow, whereas hypertonic solutions have higher solute concentration causing water to leave the cell, resulting in cell shrinkage. Understanding these distinctions is crucial in medical treatments like intravenous therapy and cellular biology studies involving osmoregulation.
Key Differences Between Tonic and Hypertonic
Tonic solutions differ from hypertonic solutions primarily in solute concentration relative to the cell's cytoplasm, with tonic solutions maintaining an equilibrium that does not cause significant water movement, while hypertonic solutions have higher solute concentrations causing water to exit the cell, leading to shrinkage. In medical and biological contexts, tonic solutions, such as isotonic saline, keep cells stable, whereas hypertonic solutions, like 3% NaCl, are used to decrease cellular swelling by drawing water out. Understanding the osmotic pressure and solute gradients is essential for applications in intravenous therapy and cellular physiology.
The Science Behind Tonicity
Tonicity refers to the ability of a solution to affect the volume and pressure of a cell through osmosis, categorized as tonic, isotonic, or hypertonic based on solute concentration relative to the cell's interior. In a tonic (often hypotonic) solution, solute concentration is lower outside the cell, causing water to flow into the cell, potentially leading to swelling or lysis. Hypertonic solutions contain higher solute concentrations than the cellular fluid, resulting in water moving out of the cell, causing it to shrink or crenate.
Osmosis: How Tonic and Hypertonic Solutions Work
Tonic and hypertonic solutions influence osmosis by the concentration of solutes relative to the cell's interior, driving water movement across the semi-permeable membrane. In a tonic (isotonic) solution, solute concentration is equal inside and outside the cell, resulting in no net water flow and maintaining cell size. Hypertonic solutions have a higher solute concentration outside the cell, causing water to move out through osmosis, leading to cell shrinkage or crenation.
Effects on Cells: Tonic vs Hypertonic
Tonic solutions influence cell volume by regulating water movement through osmosis; in a tonic solution, cells maintain their shape due to balanced solute concentrations inside and outside. Hypertonic solutions contain higher solute concentrations than the cell's interior, causing water to exit the cell, leading to cell shrinkage or crenation. This osmotic imbalance can impact cellular functions and is critical in medical treatments like intravenous therapy.
Real-World Examples of Tonic and Hypertonic Solutions
Tonic and hypertonic solutions have distinct real-world applications in medicine and biology; isotonic saline (0.9% NaCl) is commonly used for intravenous hydration to maintain fluid balance without causing cell shrinkage or swelling. Hypertonic solutions, such as 3% saline or mannitol, are utilized to reduce cerebral edema or increase blood osmolarity, drawing water out of cells to relieve intracranial pressure. In agricultural practices, hypertonic environments can cause plant cells to lose water, leading to wilting, while tonic solutions help maintain cellular turgor and plant health.
Applications in Medicine and Healthcare
Tonic and hypertonic solutions play crucial roles in medicine and healthcare, primarily in fluid therapy and cellular treatment. Tonic solutions, such as isotonic saline, maintain cell hydration without causing osmotic stress, making them ideal for intravenous hydration and electrolyte balance. Hypertonic solutions, like hypertonic saline or mannitol, are used to reduce cerebral edema and treat hyponatremia by drawing water out of cells, thereby reducing intracranial pressure and restoring electrolyte homeostasis.
Tonicity’s Role in Biology and Physiology
Tonicity is a crucial factor in biology and physiology, describing the relative concentration of solutes in extracellular fluids compared to the intracellular environment, which directly influences cell volume and function. Tonic solutions are categorized as isotonic, hypotonic, or hypertonic based on their osmotic pressure relative to cells, with hypertonic solutions causing water to exit cells, leading to cell shrinkage, while hypotonic solutions result in water influx and potential cell swelling. Understanding tonicity is essential for managing fluid balance in medical treatments, cellular homeostasis, and physiological processes such as blood pressure regulation and kidney function.
Common Misconceptions About Tonic and Hypertonic Solutions
Common misconceptions about tonic and hypertonic solutions include the belief that tonic solutions always cause cells to swell and hypertonic solutions always cause cells to shrink, while their actual impact depends on relative solute concentration. Another misunderstanding is that isotonic solutions are simply water, when in fact they contain specific solute concentrations that prevent net water movement across cell membranes. Clarifying these distinctions is crucial for accurately understanding cellular responses in medical treatments involving intravenous fluids.
Summary: Choosing the Right Solution
Choosing the right solution depends on the cellular context: hypotonic solutions cause cells to swell by moving water into the cell, while hypertonic solutions lead to cell shrinkage by drawing water out. Isotonic solutions maintain cell size by balancing solute concentration inside and outside the cell. Understanding the osmotic effects of tonic and hypertonic solutions is critical for medical treatments, hydration therapy, and cellular research.
Tonic Infographic
 libterm.com
libterm.com