Ferroptosis is a unique form of regulated cell death driven by iron-dependent lipid peroxidation, distinct from apoptosis and necrosis. This process plays a critical role in various diseases, including cancer, neurodegeneration, and ischemia-reperfusion injury, highlighting its therapeutic potential. Discover how understanding ferroptosis can advance your knowledge of cellular health and disease by exploring the full article.
Table of Comparison
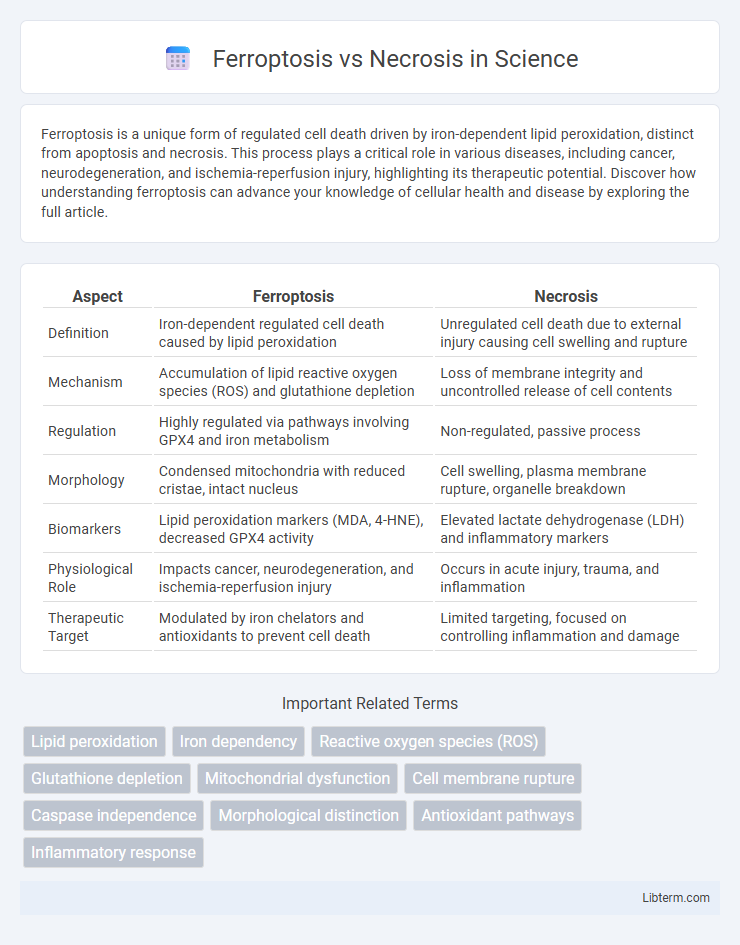
| Aspect | Ferroptosis | Necrosis |
|---|---|---|
| Definition | Iron-dependent regulated cell death caused by lipid peroxidation | Unregulated cell death due to external injury causing cell swelling and rupture |
| Mechanism | Accumulation of lipid reactive oxygen species (ROS) and glutathione depletion | Loss of membrane integrity and uncontrolled release of cell contents |
| Regulation | Highly regulated via pathways involving GPX4 and iron metabolism | Non-regulated, passive process |
| Morphology | Condensed mitochondria with reduced cristae, intact nucleus | Cell swelling, plasma membrane rupture, organelle breakdown |
| Biomarkers | Lipid peroxidation markers (MDA, 4-HNE), decreased GPX4 activity | Elevated lactate dehydrogenase (LDH) and inflammatory markers |
| Physiological Role | Impacts cancer, neurodegeneration, and ischemia-reperfusion injury | Occurs in acute injury, trauma, and inflammation |
| Therapeutic Target | Modulated by iron chelators and antioxidants to prevent cell death | Limited targeting, focused on controlling inflammation and damage |
Introduction to Ferroptosis and Necrosis
Ferroptosis is a regulated cell death mechanism characterized by iron-dependent lipid peroxidation leading to membrane damage, distinct from traditional forms of cell death. Necrosis involves uncontrolled cell swelling and rupture, typically resulting from acute cellular injury, causing inflammation in surrounding tissues. Understanding the molecular pathways of ferroptosis and necrosis reveals critical differences in cell fate determination and potential therapeutic targets in diseases such as cancer and neurodegeneration.
Defining Ferroptosis: Key Features
Ferroptosis is a distinct form of regulated cell death characterized by iron-dependent lipid peroxidation and the accumulation of reactive oxygen species, differentiating it from necrosis, which involves uncontrolled cell lysis and inflammation. Key features of ferroptosis include the depletion of glutathione, inactivation of glutathione peroxidase 4 (GPX4), and mitochondrial morphological changes such as smaller size and increased membrane density. Unlike necrosis, ferroptosis occurs through a highly regulated biochemical process, making it a target for therapeutic intervention in conditions like cancer and neurodegeneration.
Understanding Necrosis: Main Characteristics
Necrosis is a form of unregulated cell death characterized by cell membrane rupture, swelling of organelles, and subsequent inflammation in surrounding tissues. It results from external factors such as trauma, infection, or ischemia, causing irreversible damage to cellular components and loss of membrane integrity. Unlike ferroptosis, necrosis does not involve iron-dependent lipid peroxidation but leads to the release of damage-associated molecular patterns (DAMPs) that trigger immune responses.
Molecular Mechanisms of Ferroptosis
Ferroptosis is characterized by iron-dependent lipid peroxidation triggered by the accumulation of reactive oxygen species and depletion of glutathione, primarily regulated by glutathione peroxidase 4 (GPX4) inhibition. In contrast to necrosis, which involves uncontrolled cell swelling and rupture, ferroptosis is a regulated form of cell death driven by metabolic imbalances in iron homeostasis and lipid metabolism. Key molecular pathways in ferroptosis include the Fenton reaction-mediated generation of free radicals and the failure of antioxidant defenses, leading to selective peroxidation of polyunsaturated fatty acid-containing phospholipids in cell membranes.
Biochemical Pathways of Necrosis
Necrosis involves a series of biochemical pathways characterized by the loss of plasma membrane integrity, ATP depletion, and uncontrolled ion influx, leading to mitochondrial dysfunction and cell swelling. Key molecular events include the activation of calpains and lysosomal enzymes, reactive oxygen species (ROS) generation, and the release of damage-associated molecular patterns (DAMPs). This process contrasts with ferroptosis, which is iron-dependent and driven by lipid peroxidation rather than the catastrophic energy failure observed in necrosis.
Morphological Differences Between Ferroptosis and Necrosis
Ferroptosis is characterized by smaller mitochondria with increased membrane density, reduced or vanished mitochondrial cristae, and lack of typical necrotic swelling or rupture. Necrosis typically presents with cell swelling, plasma membrane rupture, and organelle breakdown leading to inflammatory responses. These distinct morphological features allow differentiation of ferroptosis, a regulated iron-dependent cell death, from necrosis, which is often uncontrolled and associated with pathological damage.
Triggers and Inducers: What Initiates Each Process?
Ferroptosis is primarily triggered by the accumulation of iron-dependent lipid peroxides, which disrupt cellular membranes and lead to oxidative cell death, often induced by glutathione depletion or inhibition of glutathione peroxidase 4 (GPX4). Necrosis is initiated by factors causing severe cellular damage such as trauma, infection, or ischemia, resulting in loss of membrane integrity and uncontrolled release of intracellular contents. While ferroptosis relies on metabolic and oxidative stress pathways, necrosis is typically a consequence of external physical or chemical injury.
Implications in Disease: Clinical Relevance
Ferroptosis, characterized by iron-dependent lipid peroxidation, plays a crucial role in neurodegenerative diseases, cancer, and ischemia-reperfusion injury, offering novel therapeutic targets distinct from necrosis. Necrosis, marked by uncontrolled cell lysis and inflammation, is a key factor in acute tissue damage and chronic inflammatory diseases, often exacerbating disease progression. Understanding the molecular mechanisms differentiating ferroptosis and necrosis enhances precision in developing disease-specific interventions and improving patient outcomes.
Therapeutic Potential and Drug Development
Ferroptosis, an iron-dependent form of regulated cell death characterized by lipid peroxidation, offers promising therapeutic potential in treating cancers and neurodegenerative diseases by selectively targeting malignant or dysfunctional cells. Necrosis, typically a form of uncontrolled cell death causing inflammation, presents challenges for drug development due to its nonspecific nature and detrimental tissue damage. Advances in ferroptosis-inducing agents and inhibitors have accelerated drug discovery efforts, aiming to exploit this pathway for oncology and neuroprotection, contrasting with limited pharmacological interventions available for modulating necrosis.
Future Perspectives: Bridging the Gap
Advancements in understanding ferroptosis and necrosis open new avenues for targeted therapies in neurodegenerative diseases and cancer treatment. Integrating molecular biomarkers with innovative drug delivery systems promises to enhance specificity and efficacy in modulating these cell death pathways. Future research should focus on elucidating the crosstalk mechanisms and developing combinatory approaches to bridge the functional gap between ferroptosis and necrosis.
Ferroptosis Infographic
 libterm.com
libterm.com