Evaporation is a crucial process where liquids transform into vapor, playing a vital role in the water cycle and climate regulation. This phenomenon occurs when molecules at the surface gain enough energy to escape into the air, influencing humidity and temperature. Discover how understanding evaporation can impact Your environment and daily life by exploring the details in this article.
Table of Comparison
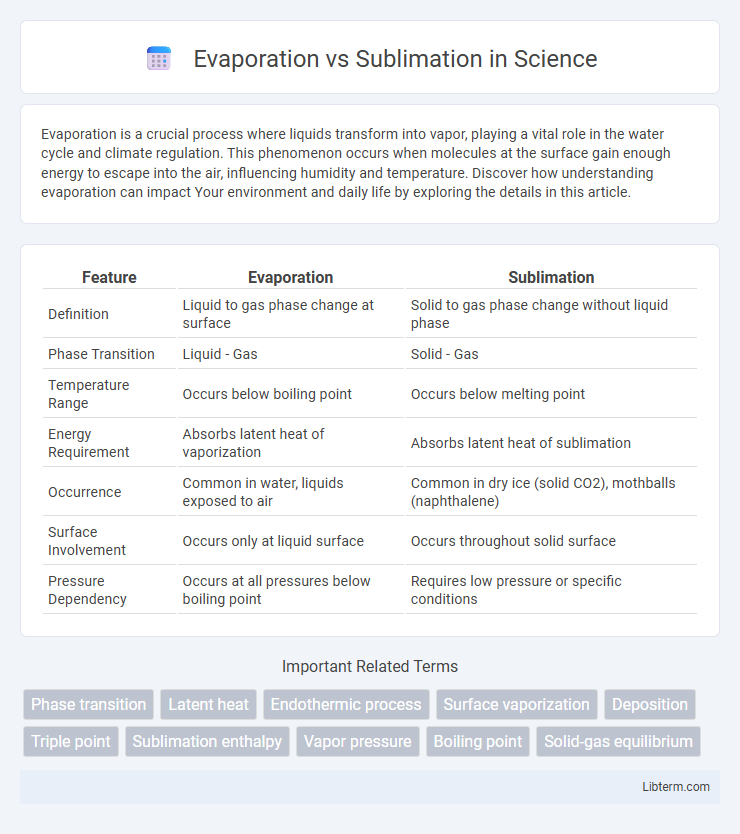
| Feature | Evaporation | Sublimation |
|---|---|---|
| Definition | Liquid to gas phase change at surface | Solid to gas phase change without liquid phase |
| Phase Transition | Liquid - Gas | Solid - Gas |
| Temperature Range | Occurs below boiling point | Occurs below melting point |
| Energy Requirement | Absorbs latent heat of vaporization | Absorbs latent heat of sublimation |
| Occurrence | Common in water, liquids exposed to air | Common in dry ice (solid CO2), mothballs (naphthalene) |
| Surface Involvement | Occurs only at liquid surface | Occurs throughout solid surface |
| Pressure Dependency | Occurs at all pressures below boiling point | Requires low pressure or specific conditions |
Introduction to Evaporation and Sublimation
Evaporation is the process where liquid molecules at the surface gain enough energy to transition into the gas phase below boiling point, commonly observed in water drying. Sublimation occurs when a solid changes directly into a gas without passing through the liquid phase, exemplified by dry ice turning into carbon dioxide vapor. Both processes involve phase changes driven by energy transfer but differ in initial and final states of matter.
Defining Evaporation: Process and Examples
Evaporation is the process where liquid water molecules gain enough energy to transform into vapor at temperatures below boiling point, commonly seen in drying puddles or wet clothes. This phase change occurs at the liquid's surface as molecules escape into the air, influenced by factors like temperature, humidity, and surface area. Unlike sublimation, evaporation involves the transition from liquid to gas without passing through the solid phase.
Understanding Sublimation: Key Concepts
Sublimation is the process where a solid changes directly into a gas without passing through the liquid phase, occurring under specific temperature and pressure conditions. This phase transition is driven by the substance's vapor pressure surpassing atmospheric pressure at temperatures below its melting point. Common examples include dry ice (solid carbon dioxide) and snow turning directly into water vapor, illustrating key principles of thermodynamics and phase behavior.
Fundamental Differences Between Evaporation and Sublimation
Evaporation is the process where a liquid changes into a gas at temperatures below boiling, occurring only at the surface, while sublimation is the direct transition of a solid into a gas without passing through the liquid phase. Evaporation requires heat energy to overcome intermolecular forces in the liquid, whereas sublimation involves breaking bonds in the solid state, often observed under specific temperature and pressure conditions. The fundamental difference lies in the phase transition pathways and the states involved: evaporation transitions liquid to vapor, sublimation transitions solid to vapor.
Physical Conditions for Evaporation
Evaporation occurs when molecules at the surface of a liquid gain enough kinetic energy to transition into the gas phase, primarily influenced by temperature, surface area, humidity, and air movement. Higher temperatures increase molecular energy, while lower humidity and increased airflow facilitate faster evaporation rates by removing vapor molecules from the liquid's surface. Unlike sublimation, which involves a solid turning directly into gas, evaporation strictly pertains to the liquid-to-gas phase change under specific physical conditions.
Physical Conditions for Sublimation
Sublimation occurs when a solid changes directly into a gas without passing through the liquid phase, typically under low pressure and temperatures below the substance's melting point. This phase transition requires the vapor pressure of the solid to equal the surrounding pressure, often happening in vacuum or dry, cold environments. Substances like dry ice (solid CO2) and iodine exhibit sublimation under these specific physical conditions.
Real-World Examples of Evaporation
Evaporation is the process where liquid water transforms into vapor, commonly seen when puddles dry after rain or wet clothes dry in the sun. This phase change occurs at the surface of the liquid and is influenced by temperature, humidity, and airflow, exemplified by water evaporating from oceans and lakes, contributing to the natural water cycle. Unlike sublimation, which involves a direct solid-to-gas transition, evaporation is a key mechanism in real-world applications such as cooling systems, agriculture irrigation, and climate regulation.
Real-World Examples of Sublimation
Sublimation occurs when a solid transitions directly to a gas without passing through the liquid state, as seen in dry ice (solid carbon dioxide) turning into carbon dioxide gas at room temperature. Another real-world example is the sublimation of mothballs, where naphthalene solid vaporizes to repel insects. Sublimation is utilized in freeze-drying processes, where ice in frozen food converts directly into water vapor, preserving the food's texture and nutrients.
Industrial and Practical Applications
Evaporation plays a crucial role in industries such as chemical manufacturing and wastewater treatment by enabling efficient separation and concentration of liquids through controlled heating. Sublimation is extensively used in freeze-drying pharmaceuticals and preserving perishable food products, leveraging the direct solid-to-gas transition to maintain material integrity. Both processes optimize production efficiency and product quality by utilizing phase changes under specific temperature and pressure conditions.
Summary: Evaporation vs Sublimation Comparison
Evaporation is the phase transition where a liquid turns into vapor at temperatures below its boiling point, primarily occurring at the surface, while sublimation is the direct transition of a solid into a gas without passing through the liquid phase. Evaporation depends on temperature, surface area, and humidity, whereas sublimation is influenced by temperature and pressure conditions, often observed in substances like dry ice and snow. Both processes involve energy absorption but differ in phase changes and environmental requirements.
Evaporation Infographic
 libterm.com
libterm.com