Crystallization is a vital process in chemistry and materials science where a solid forms from a solution or melt, resulting in a highly ordered and structured crystal lattice. This phenomenon is essential for purifying substances and creating materials with specific properties, influencing industries from pharmaceuticals to manufacturing. Explore the detailed mechanisms and applications of crystallization in the following article to enhance your understanding.
Table of Comparison
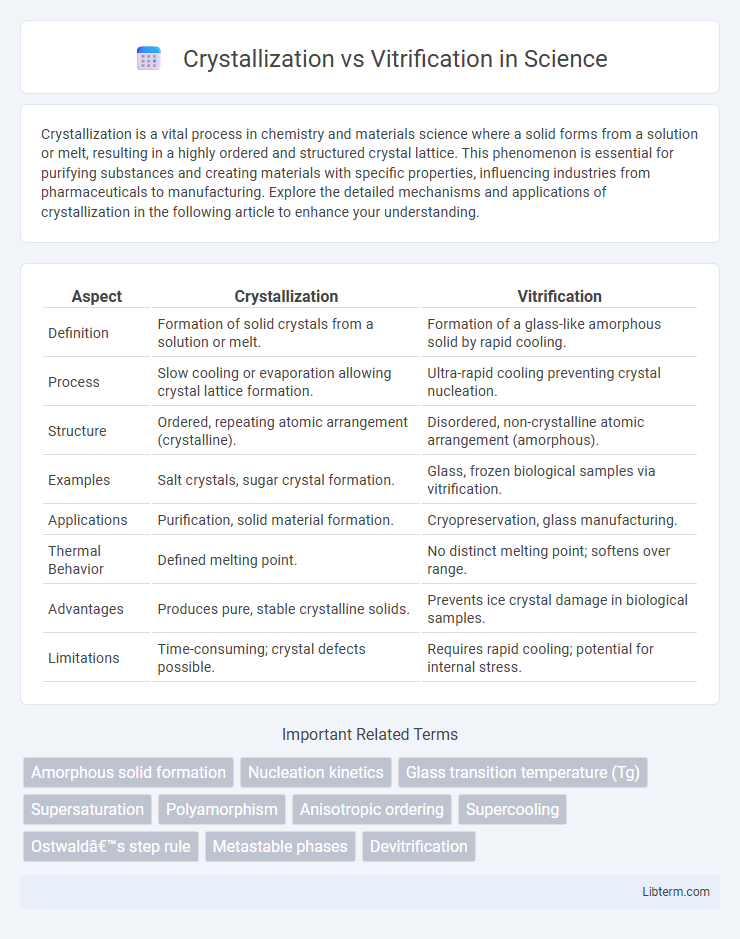
| Aspect | Crystallization | Vitrification |
|---|---|---|
| Definition | Formation of solid crystals from a solution or melt. | Formation of a glass-like amorphous solid by rapid cooling. |
| Process | Slow cooling or evaporation allowing crystal lattice formation. | Ultra-rapid cooling preventing crystal nucleation. |
| Structure | Ordered, repeating atomic arrangement (crystalline). | Disordered, non-crystalline atomic arrangement (amorphous). |
| Examples | Salt crystals, sugar crystal formation. | Glass, frozen biological samples via vitrification. |
| Applications | Purification, solid material formation. | Cryopreservation, glass manufacturing. |
| Thermal Behavior | Defined melting point. | No distinct melting point; softens over range. |
| Advantages | Produces pure, stable crystalline solids. | Prevents ice crystal damage in biological samples. |
| Limitations | Time-consuming; crystal defects possible. | Requires rapid cooling; potential for internal stress. |
Introduction to Crystallization and Vitrification
Crystallization involves the formation of highly ordered, repeating molecular structures that result in solid crystals, commonly observed in natural processes like ice formation and industrial applications such as pharmaceutical drug production. Vitrification refers to the rapid cooling of a substance to form an amorphous, glass-like solid without crystallization, preserving the disordered molecular arrangement found in liquids. Both processes are critical in materials science and cryopreservation, influencing the physical properties and stability of substances.
Defining Crystallization: Process and Principles
Crystallization is the process by which a substance transitions from a liquid or solution state into a highly ordered, solid crystalline structure through nucleation and subsequent growth of crystal lattices. This process relies on principles of thermodynamics and kinetics, where supersaturation or supercooling drives the formation of stable nuclei that act as a template for orderly molecular arrangement. Crystallization is widely utilized in chemical synthesis, material science, and pharmaceutical manufacturing to achieve purity, control particle size, and enhance material properties.
Understanding Vitrification: What Sets It Apart
Vitrification is a rapid cooling process that transforms a liquid into a glass-like, amorphous solid without forming ice crystals, contrasting with crystallization where structured ice crystals develop. This method is crucial in cryopreservation, especially for biological samples like embryos and oocytes, because it prevents cellular damage caused by ice crystals. The unique ability of vitrification to maintain cellular integrity and viability makes it a preferred technique in reproductive medicine and tissue preservation.
Key Differences Between Crystallization and Vitrification
Crystallization involves the formation of organized, repeating atomic or molecular structures resulting in solid crystals, whereas vitrification produces an amorphous, glass-like solid lacking long-range order. Crystallization typically occurs through nucleation and growth processes under slow cooling or solvent evaporation, while vitrification requires rapid cooling or the use of cryoprotectants to prevent crystal formation. These fundamental differences impact material properties such as transparency, mechanical strength, and thermal stability, influencing applications in fields like cryopreservation, materials science, and pharmaceuticals.
Mechanisms: How Crystallization Occurs
Crystallization occurs through the nucleation and growth of ordered molecular structures as a liquid cools or a solution becomes supersaturated, leading to the formation of a crystalline solid. The process begins with the formation of stable nuclei that serve as templates for additional molecules, promoting an organized lattice arrangement. This mechanism contrasts with vitrification, where rapid cooling prevents nucleation, resulting in an amorphous, glass-like solid without long-range order.
Mechanisms: The Science Behind Vitrification
Vitrification is a rapid cooling process that transforms a liquid into a glass-like amorphous solid without forming ice crystals, unlike crystallization which involves the orderly arrangement of molecules into a rigid crystalline structure. The mechanism behind vitrification relies on bypassing the nucleation and growth phases of crystallization by increasing the viscosity so quickly that molecular mobility is severely restricted, preventing ice crystal formation. This glass transition preserves cellular integrity, making vitrification a superior technique in cryopreservation and biostorage applications.
Applications of Crystallization in Industry and Research
Crystallization plays a critical role in industries such as pharmaceuticals, where it is used to purify and isolate active pharmaceutical ingredients, ensuring consistent drug efficacy and stability. In chemical manufacturing, crystallization enables the production of high-purity compounds and controls particle size, impacting product quality and downstream processing. Research applications leverage crystallization to study molecular structures and develop novel materials with tailored properties, enhancing innovation in nanotechnology and materials science.
Uses of Vitrification in Science and Medicine
Vitrification preserves biological samples by rapidly cooling them to prevent ice crystal formation, making it essential for cryopreservation of embryos, oocytes, and tissues in reproductive medicine and regenerative therapies. This technique enhances cell viability and reduces damage compared to traditional crystallization methods, improving success rates in in vitro fertilization (IVF) and organ transplantation. Vitrification also plays a critical role in preserving biological specimens for research, enabling long-term storage without loss of structural integrity.
Comparative Advantages and Limitations
Crystallization offers precise molecular alignment and stable crystal formation but risks ice crystal damage to cell structures, limiting its use in biological preservation. Vitrification rapidly solidifies liquids into a glass-like state without crystal formation, minimizing structural damage and enhancing cell viability, yet it requires ultra-fast cooling rates and high cryoprotectant concentrations, posing toxicity challenges. The choice between crystallization and vitrification depends on the balance between structural integrity preservation and practical application feasibility in cryopreservation techniques.
Choosing Between Crystallization and Vitrification
Choosing between crystallization and vitrification depends on the specific characteristics of the material and desired outcomes in cryopreservation or material processing. Crystallization is preferred when controlled crystal growth can enhance material properties or preserve biological samples with minimal damage. Vitrification is optimal for preventing ice formation entirely, ensuring amorphous solidification ideal for samples sensitive to ice-induced cellular damage, such as oocytes and embryos.
Crystallization Infographic
 libterm.com
libterm.com