Corrosion gradually deteriorates metals through chemical reactions with their environment, compromising structural integrity and safety. Effective prevention methods such as coatings, cathodic protection, and material selection are essential to extend the lifespan of metallic components. Explore the rest of the article to understand how corrosion affects your materials and learn best practices for protection.
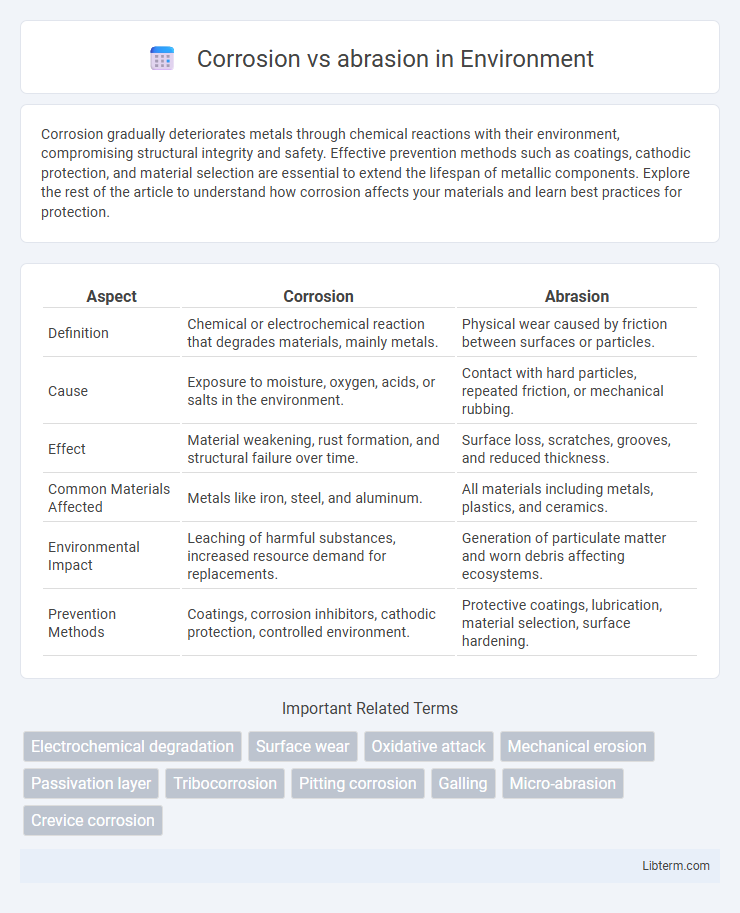
Table of Comparison
| Aspect | Corrosion | Abrasion |
|---|---|---|
| Definition | Chemical or electrochemical reaction that degrades materials, mainly metals. | Physical wear caused by friction between surfaces or particles. |
| Cause | Exposure to moisture, oxygen, acids, or salts in the environment. | Contact with hard particles, repeated friction, or mechanical rubbing. |
| Effect | Material weakening, rust formation, and structural failure over time. | Surface loss, scratches, grooves, and reduced thickness. |
| Common Materials Affected | Metals like iron, steel, and aluminum. | All materials including metals, plastics, and ceramics. |
| Environmental Impact | Leaching of harmful substances, increased resource demand for replacements. | Generation of particulate matter and worn debris affecting ecosystems. |
| Prevention Methods | Coatings, corrosion inhibitors, cathodic protection, controlled environment. | Protective coatings, lubrication, material selection, surface hardening. |
Introduction to Corrosion and Abrasion
Corrosion is the chemical or electrochemical reaction between materials, typically metals, and their environment, leading to material degradation and loss of functionality. Abrasion involves the mechanical wearing or rubbing away of a surface caused by friction between the material and moving particles or surfaces. Understanding the distinct mechanisms of corrosion and abrasion is essential for selecting appropriate materials and protective measures in industrial and environmental applications.
Defining Corrosion
Corrosion is the chemical or electrochemical reaction between a material, usually metal, and its environment, leading to the gradual deterioration of the material's properties. This process often results in rust formation on iron and steel, caused by oxidation in the presence of moisture and oxygen. Unlike abrasion, which involves physical wear from friction, corrosion is driven by chemical interactions that break down the material at the molecular level.
Understanding Abrasion
Abrasion involves the mechanical wearing away of material surfaces due to friction from contact with other materials, often resulting in surface scratches, grooves, or material loss. It fundamentally differs from corrosion, which is a chemical or electrochemical process leading to material degradation through reactions with environmental agents like moisture, oxygen, or chemicals. Understanding abrasion emphasizes selecting wear-resistant materials and coatings that minimize surface damage caused by mechanical interactions in industrial applications.
Key Differences Between Corrosion and Abrasion
Corrosion is a chemical or electrochemical process causing material degradation through reactions with environmental elements like oxygen and moisture, while abrasion involves mechanical wear caused by friction or rubbing against rough surfaces. Corrosion often leads to rust or oxide layer formation, predominantly affecting metals, whereas abrasion results in surface scratches, grooves, or material loss without chemical change. The rate of corrosion depends on environmental factors such as humidity and pH, whereas abrasion depends on physical factors like contact pressure, surface hardness, and relative motion.
Causes of Corrosion
Corrosion primarily occurs due to chemical reactions between materials, usually metals, and their environment, often involving moisture, oxygen, acids, or salts. Electrochemical processes, such as oxidation, are key drivers, where metal atoms lose electrons and form oxides or other compounds. Factors like pH levels, temperature, and the presence of electrolytes can accelerate the corrosion rate, distinguishing it from abrasion, which is physical wear caused by friction.
Causes of Abrasion
Abrasion primarily results from mechanical action where hard particles or rough surfaces repeatedly rub against a material, causing surface wear and material loss. Common causes include friction from moving parts, contact with abrasive materials such as sand or grit, and the presence of contaminants that increase friction. Unlike corrosion, which is a chemical or electrochemical process, abrasion is purely a physical degradation driven by mechanical forces.
Effects on Materials and Structural Integrity
Corrosion chemically degrades materials by breaking down their molecular structure, leading to pitting, rust, and loss of metal thickness, which compromises structural integrity over time. Abrasion physically wears away surface layers through friction or contact with hard particles, causing surface roughness and thinning that reduce load-bearing capacity and increase susceptibility to fatigue. Both processes accelerate material failure, but corrosion typically leads to internal weakening, while abrasion primarily causes surface damage.
Prevention and Protection Methods
Corrosion prevention involves using protective coatings like paint, galvanization, or anodizing to create a barrier against chemical reactions with the environment, while abrasion protection relies on hard, wear-resistant materials such as ceramics, rubber linings, or metal alloys to resist mechanical wear. Implementing cathodic protection systems and corrosion inhibitors significantly reduces metal deterioration, whereas abrasion can be minimized through surface hardening techniques like carburizing or applying sacrificial wear plates. Regular maintenance and monitoring with advanced sensors ensure early detection and prolong the lifespan of structures exposed to both corrosion and abrasion.
Industrial Applications and Real-World Examples
Corrosion and abrasion impact industrial equipment differently, with corrosion causing chemical degradation often seen in pipelines exposed to harsh chemicals, while abrasion results from mechanical wear such as conveyor belts rubbing against surfaces in mining operations. Steel structures in offshore oil rigs face severe corrosion due to saltwater, whereas abrasion is prominent in cement plants where materials constantly grind against machinery. Understanding these differences enables industries to select appropriate protective coatings and maintenance schedules to prolong equipment life and ensure operational efficiency.
Conclusion: Choosing the Right Mitigation Strategy
Effective mitigation of corrosion and abrasion hinges on understanding their distinct mechanisms--corrosion involves chemical or electrochemical reactions deteriorating material surfaces, while abrasion results from mechanical wear due to friction or impact. Selecting appropriate protective measures, such as corrosion-resistant coatings, cathodic protection for corrosion, or wear-resistant materials and surface treatments for abrasion, ensures durability and cost-efficiency. Tailoring strategies to specific environmental conditions and operational stresses enhances asset longevity and performance.
Corrosion Infographic
 libterm.com
libterm.com