Mineralization is the process where organic material is decomposed into inorganic minerals, enriching soil fertility and supporting plant growth. This transformation plays a crucial role in nutrient cycling and ecosystem sustainability. Discover how mineralization impacts your garden and natural environments by reading the rest of the article.
Table of Comparison
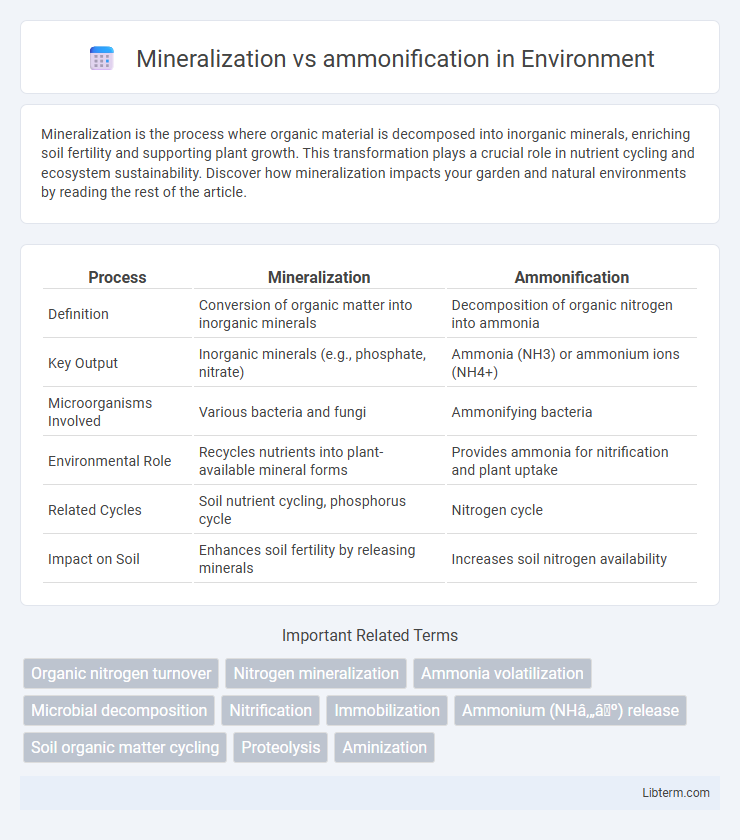
| Process | Mineralization | Ammonification |
|---|---|---|
| Definition | Conversion of organic matter into inorganic minerals | Decomposition of organic nitrogen into ammonia |
| Key Output | Inorganic minerals (e.g., phosphate, nitrate) | Ammonia (NH3) or ammonium ions (NH4+) |
| Microorganisms Involved | Various bacteria and fungi | Ammonifying bacteria |
| Environmental Role | Recycles nutrients into plant-available mineral forms | Provides ammonia for nitrification and plant uptake |
| Related Cycles | Soil nutrient cycling, phosphorus cycle | Nitrogen cycle |
| Impact on Soil | Enhances soil fertility by releasing minerals | Increases soil nitrogen availability |
Understanding Mineralization and Ammonification
Mineralization is the microbial process that converts organic nitrogen compounds into inorganic forms such as ammonium, making nitrogen available for plant uptake. Ammonification specifically refers to the decomposition of organic nitrogen in dead organisms and waste into ammonia or ammonium ions by microbes. Understanding these processes is crucial for nutrient cycling in soil, influencing soil fertility and ecosystem productivity.
Key Differences Between Mineralization and Ammonification
Mineralization converts organic nitrogen in dead matter into inorganic forms, mainly ammonium (NH4+), while ammonification specifically refers to the microbial decomposition process producing ammonia (NH3) or ammonium ions from organic nitrogen compounds. Mineralization encompasses a broader scope of nutrient transformation, including carbon and nitrogen compounds, whereas ammonification targets nitrogen compounds alone. Key differences include their roles in the nitrogen cycle, with mineralization facilitating nutrient availability and ammonification serving as a crucial intermediate step in nitrogen turnover by soil microbes.
The Role of Microorganisms in Both Processes
Microorganisms play a crucial role in both mineralization and ammonification by breaking down organic matter to recycle nutrients. In mineralization, bacteria and fungi decompose complex organic compounds into inorganic minerals like nitrate and phosphate, making essential nutrients available for plant uptake. During ammonification, specific bacteria convert organic nitrogen from dead organisms and waste into ammonia, which serves as a key step in the nitrogen cycle.
Stages of Nitrogen Transformation in Soil
Mineralization is the microbial process in soil where organic nitrogen compounds are decomposed into inorganic forms, primarily ammonium (NH4+), making nitrogen available to plants. Ammonification specifically refers to the conversion of organic nitrogen from dead plant and animal matter into ammonia (NH3) or ammonium through microbial activity. These stages are crucial components of nitrogen transformation in soil, facilitating nutrient cycling and enhancing soil fertility by converting complex organic nitrogen into accessible inorganic nitrogen forms.
The Biochemical Pathways of Mineralization
Mineralization in soil involves the biochemical breakdown of organic nitrogen compounds into inorganic forms like ammonium (NH4+), driven primarily by microbial enzymes such as proteases and deaminases that sequentially degrade proteins into amino acids and then ammonia. This process contrasts with ammonification, which specifically refers to the conversion of organic nitrogen into ammonium, but mineralization encompasses a broader spectrum of nutrient releases including phosphorus and sulfur. Enzymatic pathways in mineralization include the oxidative deamination of amino acids, releasing ammonia that becomes available for nitrification, influencing soil fertility and nutrient cycling in ecosystems.
Ammonification: Formation and Importance of Ammonia
Ammonification is the microbial process that converts organic nitrogen from dead plants and animals into ammonia, playing a critical role in the nitrogen cycle by recycling nitrogen within ecosystems. Ammonia produced during ammonification serves as a vital nitrogen source for nitrifying bacteria, which subsequently convert it into nitrites and nitrates essential for plant nutrition. This process ensures the availability of inorganic nitrogen, supporting soil fertility and promoting sustainable agricultural productivity.
Environmental Factors Influencing Each Process
Mineralization rates are significantly affected by soil temperature, moisture, and pH, with warmer and moist conditions accelerating the breakdown of organic matter into inorganic nutrients. Ammonification is influenced by the presence of decomposer microbes, substrate composition, and oxygen availability, where aerobic conditions generally enhance the conversion of organic nitrogen to ammonia. Both processes are sensitive to environmental stressors such as heavy metals, salinity, and soil texture, which can inhibit microbial activity and nutrient cycling efficiency.
Impact on Soil Fertility and Crop Productivity
Mineralization converts organic nitrogen compounds into inorganic forms like ammonium, which enhances soil fertility by making nitrogen readily available for plant uptake, directly boosting crop productivity. Ammonification, a subset of mineralization, specifically transforms organic nitrogen into ammonium through microbial decomposition, maintaining a steady nitrogen supply essential for healthy plant growth. Efficient mineralization and ammonification processes improve nitrogen cycling, resulting in higher nutrient availability, improved soil structure, and sustained agricultural output.
Significance in the Nitrogen Cycle
Mineralization converts organic nitrogen into ammonium, making nitrogen accessible to plants and soil microbes, thus playing a crucial role in nutrient availability within the nitrogen cycle. Ammonification specifically breaks down nitrogenous organic matter into ammonia or ammonium ions, facilitating subsequent nitrification processes that replenish soil nitrogen content. Both processes are vital for maintaining soil fertility and supporting ecosystem productivity by ensuring continuous nitrogen turnover.
Practical Implications for Sustainable Agriculture
Mineralization converts organic nitrogen into inorganic forms like ammonium, making nutrients immediately available for plant uptake and enhancing soil fertility. Ammonification, a key step in mineralization, breaks down organic nitrogen into ammonia, which soil microbes further process into plant-accessible forms, supporting nutrient cycling. Efficient management of these processes improves nitrogen use efficiency, reduces chemical fertilizer dependence, and promotes sustainable agricultural practices by maintaining soil health and optimizing crop yield.
Mineralization Infographic
 libterm.com
libterm.com