Nitrogen fixation converts atmospheric nitrogen into forms plants can absorb, playing a crucial role in agriculture and ecosystem health. This natural process supports soil fertility and reduces the need for synthetic fertilizers. Discover how nitrogen fixation impacts your environment and the methods used to enhance it in the rest of this article.
Table of Comparison
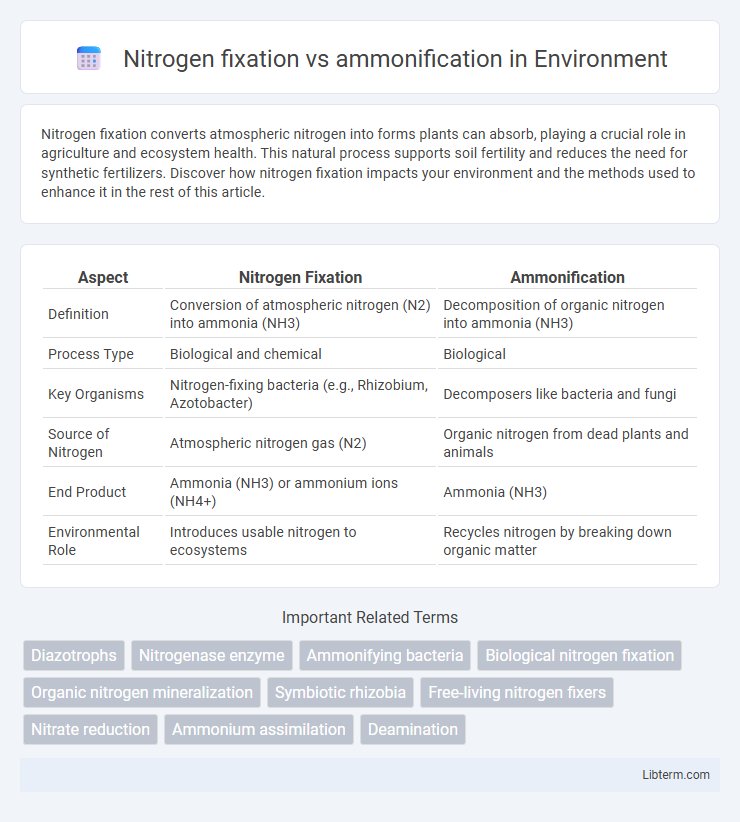
| Aspect | Nitrogen Fixation | Ammonification |
|---|---|---|
| Definition | Conversion of atmospheric nitrogen (N2) into ammonia (NH3) | Decomposition of organic nitrogen into ammonia (NH3) |
| Process Type | Biological and chemical | Biological |
| Key Organisms | Nitrogen-fixing bacteria (e.g., Rhizobium, Azotobacter) | Decomposers like bacteria and fungi |
| Source of Nitrogen | Atmospheric nitrogen gas (N2) | Organic nitrogen from dead plants and animals |
| End Product | Ammonia (NH3) or ammonium ions (NH4+) | Ammonia (NH3) |
| Environmental Role | Introduces usable nitrogen to ecosystems | Recycles nitrogen by breaking down organic matter |
Introduction to Nitrogen Fixation and Ammonification
Nitrogen fixation is a crucial biological process where atmospheric nitrogen (N2) is converted into ammonia (NH3) by specialized bacteria such as Rhizobium and Azotobacter, enabling plants to access essential nitrogen for growth. Ammonification involves the decomposition of organic nitrogen from dead organisms and waste products into ammonia by saprophytic microorganisms, replenishing soil nitrogen. Together, these processes sustain the nitrogen cycle by transforming inert nitrogen compounds into bioavailable forms essential for ecosystem productivity.
Nitrogen Cycle Overview
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen accessible to plants and microbes, while ammonification breaks down organic nitrogen in dead organisms and waste into ammonia, returning it to the soil. Both processes are essential stages in the nitrogen cycle, facilitating nitrogen availability for biological uptake and maintaining soil fertility. Nitrogen fixation is primarily driven by bacteria such as Rhizobium in symbiosis with legumes, whereas ammonification involves decomposer microbes that recycle organic nitrogen compounds.
What is Nitrogen Fixation?
Nitrogen fixation is the biological process in which atmospheric nitrogen (N2) is converted into ammonia (NH3) by specialized microorganisms such as Rhizobium bacteria and cyanobacteria. This conversion is crucial because atmospheric nitrogen is inert and unavailable directly to plants, whereas ammonia serves as a bioavailable form of nitrogen essential for synthesizing amino acids and nucleotides. Nitrogen fixation significantly contributes to soil fertility by replenishing nitrogen levels and supporting plant growth, distinguishing it from ammonification, where organic nitrogen compounds decompose to release ammonia back into the soil.
Key Organisms Involved in Nitrogen Fixation
Nitrogen fixation primarily involves diazotrophic bacteria such as Rhizobium, which form symbiotic relationships with leguminous plants, and free-living cyanobacteria like Anabaena. These key organisms convert atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen accessible for microbial and plant use. In contrast, ammonification is carried out by decomposer bacteria and fungi that break down organic nitrogen from dead organisms into ammonia, highlighting the distinct microbial processes in nitrogen cycling.
Ammonification Defined
Ammonification is the microbial process that converts organic nitrogen from dead organisms and waste products into ammonia or ammonium ions, facilitating nitrogen recycling in ecosystems. This process is crucial for maintaining soil fertility by making nitrogen available to plants in a usable form. Unlike nitrogen fixation, which converts atmospheric nitrogen into organic forms, ammonification breaks down existing organic nitrogen compounds into simpler inorganic molecules.
Microbial Agents in Ammonification
Ammonification primarily involves microbial agents such as decomposer bacteria and fungi that break down organic nitrogen compounds into ammonia, facilitating nitrogen recycling in ecosystems. Common microbial genera involved in ammonification include Bacillus, Clostridium, and Pseudomonas, which secrete enzymes like proteases and deaminases to degrade amino acids and nucleic acids. Unlike nitrogen fixation, which involves diazotrophic bacteria converting atmospheric N2 into ammonia, ammonification relies on saprophytic microbes decomposing dead organic matter to release ammonia into the soil.
Differences Between Nitrogen Fixation and Ammonification
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3) using nitrogenase enzymes, primarily performed by certain bacteria and cyanobacteria. In contrast, ammonification breaks down organic nitrogen from dead organisms and waste into ammonia or ammonium ions through microbial decomposition. While nitrogen fixation introduces usable nitrogen into ecosystems, ammonification recycles nitrogen from organic matter back into inorganic forms.
Ecological Significance of Both Processes
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen available to plants and supporting ecosystem productivity by sustaining soil fertility. Ammonification decomposes organic nitrogen from dead organisms into ammonia, recycling nitrogen within ecosystems and maintaining the nitrogen cycle's continuity. Both processes are essential for nutrient cycling, ecosystem health, and supporting biodiverse habitats.
Human Influence on Nitrogen Fixation and Ammonification
Human activities significantly impact nitrogen fixation through the extensive use of synthetic fertilizers and industrial nitrogen fixation processes like the Haber-Bosch method, which increase reactive nitrogen in ecosystems and alter natural nitrogen cycles. Ammonification is indirectly influenced by human-induced changes in soil composition, organic matter inputs from agricultural waste, and pollution, which can enhance or disrupt the microbial breakdown of organic nitrogen into ammonium. These anthropogenic effects contribute to nutrient imbalances, affecting soil fertility, greenhouse gas emissions, and water quality.
Conclusion: Balancing Nitrogen Transformations in Nature
Nitrogen fixation converts atmospheric nitrogen (N2) into bioavailable ammonia, essential for plant growth, while ammonification breaks down organic nitrogen into ammonium, recycling nitrogen within ecosystems. The balance between these processes maintains soil fertility and supports the nitrogen cycle by ensuring a continuous supply of usable nitrogen forms. Effective nitrogen management in agriculture and ecology relies on optimizing both nitrogen fixation and ammonification to sustain healthy ecosystems and promote crop productivity.
Nitrogen fixation Infographic
 libterm.com
libterm.com