Mineralization is a natural geological process where organic material transforms into minerals over time, enriching soil and rock layers with essential nutrients like calcium, phosphate, and iron. This process significantly affects the fertility of agricultural land and the quality of water resources by releasing bound elements back into the ecosystem. Discover how mineralization impacts your environment and its vital role in sustaining natural cycles throughout this article.
Table of Comparison
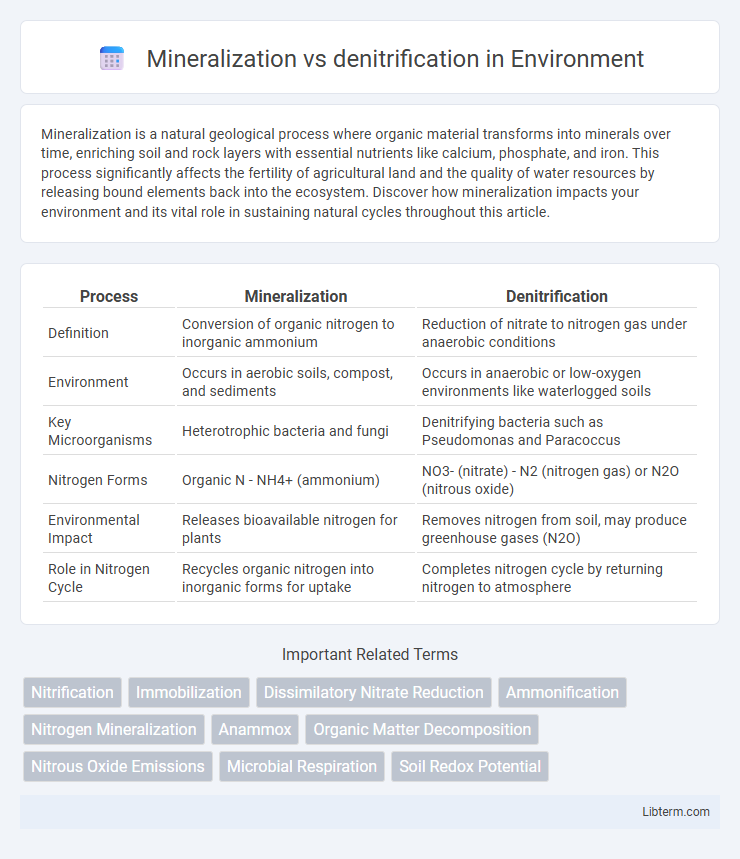
| Process | Mineralization | Denitrification |
|---|---|---|
| Definition | Conversion of organic nitrogen to inorganic ammonium | Reduction of nitrate to nitrogen gas under anaerobic conditions |
| Environment | Occurs in aerobic soils, compost, and sediments | Occurs in anaerobic or low-oxygen environments like waterlogged soils |
| Key Microorganisms | Heterotrophic bacteria and fungi | Denitrifying bacteria such as Pseudomonas and Paracoccus |
| Nitrogen Forms | Organic N - NH4+ (ammonium) | NO3- (nitrate) - N2 (nitrogen gas) or N2O (nitrous oxide) |
| Environmental Impact | Releases bioavailable nitrogen for plants | Removes nitrogen from soil, may produce greenhouse gases (N2O) |
| Role in Nitrogen Cycle | Recycles organic nitrogen into inorganic forms for uptake | Completes nitrogen cycle by returning nitrogen to atmosphere |
Introduction to Mineralization and Denitrification
Mineralization is the microbial process that converts organic nitrogen compounds into inorganic forms like ammonium, making nutrients available for plant uptake. Denitrification is a microbial respiratory process that reduces nitrate to gaseous nitrogen forms such as N2 or N2O, leading to nitrogen loss from soils and aquatic systems. Both processes play crucial roles in the nitrogen cycle by regulating nitrogen availability and emissions in terrestrial and aquatic ecosystems.
Overview of the Nitrogen Cycle
Mineralization converts organic nitrogen in soil organic matter into inorganic ammonium, making nitrogen available for plant uptake. Denitrification reduces nitrate to gaseous nitrogen forms like N2 and N2O, returning nitrogen to the atmosphere and completing the nitrogen cycle. These processes regulate nitrogen availability and influence soil fertility and greenhouse gas emissions in ecosystems.
Defining Mineralization: Key Processes
Mineralization is the microbial process that converts organic nitrogen compounds into inorganic forms such as ammonium, making nitrogen available for plant uptake. It involves the breakdown of organic matter by soil microbes, releasing essential nutrients and playing a crucial role in the nitrogen cycle. This process contrasts with denitrification, which reduces nitrate to gaseous nitrogen forms, thereby removing nitrogen from the soil ecosystem.
Understanding Denitrification: Mechanisms and Pathways
Denitrification is a microbial-driven process converting nitrate (NO3-) into nitrogen gas (N2), primarily through intermediate forms such as nitrite (NO2-), nitric oxide (NO), and nitrous oxide (N2O). This anaerobic pathway occurs in oxygen-depleted environments, where facultative bacteria use nitrate as an alternative electron acceptor, effectively reducing nitrogen availability in soils and aquatic systems. Understanding the enzymatic mechanisms involving nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase is crucial for optimizing nitrogen cycling and mitigating greenhouse gas emissions.
Microbial Roles in Mineralization and Denitrification
Microbial roles in mineralization involve decomposing organic nitrogen compounds into inorganic ammonium, which replenishes soil nitrogen availability for plants. Denitrification is driven by facultative anaerobic bacteria that convert nitrate into nitrogen gas, effectively removing bioavailable nitrogen and reducing soil fertility. These microbial processes are crucial for regulating nitrogen cycling and maintaining ecosystem nutrient balance.
Environmental Factors Influencing Each Process
Temperature, soil moisture, and pH significantly influence mineralization and denitrification rates, with mineralization favoring warmer, well-aerated soils and optimum pH levels around neutral. Denitrification intensifies in anaerobic, waterlogged conditions where nitrate availability and organic carbon serve as critical substrates. Oxygen concentration acts as a key regulator, suppressing denitrification under aerobic conditions while promoting mineralization through microbial activity.
Impacts on Soil Fertility and Crop Productivity
Mineralization converts organic nitrogen into inorganic forms like ammonium, enhancing soil fertility by increasing nutrient availability for crops. Denitrification reduces soil nitrate levels by converting nitrates to gaseous nitrogen, potentially limiting nitrogen availability and decreasing crop productivity. Balancing these processes is crucial for maintaining optimal nitrogen cycling, ensuring sustained soil fertility and maximizing agricultural yields.
Consequences for Water Quality and Ecosystems
Mineralization releases inorganic nutrients like ammonium into the soil, increasing nitrogen availability but potentially leading to nitrate leaching and eutrophication in aquatic systems. Denitrification converts nitrate to nitrogen gas, reducing nitrate concentrations and mitigating water pollution but producing nitrous oxide, a potent greenhouse gas. The balance between these processes influences nutrient cycling, water quality, and ecosystem health by controlling nitrogen forms and their environmental impacts.
Human Influences and Management Strategies
Human activities such as intensive agriculture and wastewater discharge elevate nitrogen loads, accelerating mineralization and denitrification processes in soils and aquatic systems. Management strategies emphasize optimizing fertilizer application, promoting cover cropping, and enhancing wetland restoration to balance nitrogen cycling and reduce greenhouse gas emissions. Implementing these approaches mitigates the negative effects of disrupted nitrogen transformations caused by anthropogenic disturbances.
Comparative Summary: Mineralization vs Denitrification
Mineralization converts organic nitrogen into inorganic ammonium, enhancing soil nitrogen availability, whereas denitrification transforms nitrate into nitrogen gases, reducing soil nitrogen levels and releasing N2 or N2O into the atmosphere. Mineralization primarily occurs under aerobic conditions through microbial decomposition, while denitrification is an anaerobic microbial process prevalent in waterlogged or oxygen-poor soils. These contrasting processes regulate soil nitrogen cycling, influencing nutrient availability and environmental nitrogen emissions.
Mineralization Infographic
 libterm.com
libterm.com