Nitrification is a crucial biological process where ammonia is converted into nitrate by specialized bacteria, playing a vital role in the nitrogen cycle and soil fertility. This transformation enhances nutrient availability for plants, directly impacting agricultural productivity and ecosystem health. Discover more about how nitrification affects your environment and its importance in maintaining balanced ecosystems in the rest of this article.
Table of Comparison
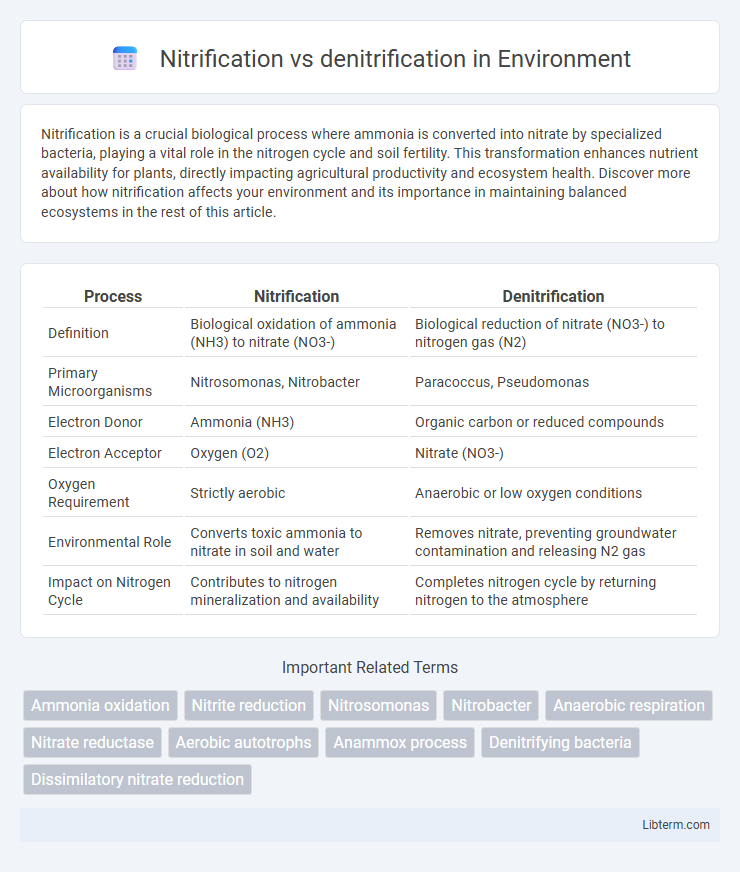
| Process | Nitrification | Denitrification |
|---|---|---|
| Definition | Biological oxidation of ammonia (NH3) to nitrate (NO3-) | Biological reduction of nitrate (NO3-) to nitrogen gas (N2) |
| Primary Microorganisms | Nitrosomonas, Nitrobacter | Paracoccus, Pseudomonas |
| Electron Donor | Ammonia (NH3) | Organic carbon or reduced compounds |
| Electron Acceptor | Oxygen (O2) | Nitrate (NO3-) |
| Oxygen Requirement | Strictly aerobic | Anaerobic or low oxygen conditions |
| Environmental Role | Converts toxic ammonia to nitrate in soil and water | Removes nitrate, preventing groundwater contamination and releasing N2 gas |
| Impact on Nitrogen Cycle | Contributes to nitrogen mineralization and availability | Completes nitrogen cycle by returning nitrogen to the atmosphere |
Introduction to Nitrification and Denitrification
Nitrification is a microbial process that converts ammonia into nitrate through oxidation, playing a crucial role in the nitrogen cycle by making nitrogen available to plants. Denitrification, in contrast, involves the reduction of nitrate to nitrogen gas by anaerobic bacteria, effectively removing bioavailable nitrogen from ecosystems and returning it to the atmosphere. These complementary processes regulate soil fertility, influence nitrogen availability, and impact environmental nitrogen balance.
Key Differences Between Nitrification and Denitrification
Nitrification is a microbial aerobic process converting ammonia (NH3) into nitrate (NO3-) through nitrite (NO2-) as an intermediate, primarily conducted by ammonia-oxidizing bacteria and nitrite-oxidizing bacteria. Denitrification is an anaerobic microbial process that reduces nitrate (NO3-) to nitrogen gas (N2), involving facultative bacteria such as Paracoccus and Pseudomonas species. The key difference lies in oxygen requirement: nitrification requires oxygen, while denitrification occurs in oxygen-depleted environments, influencing nitrogen availability in soil and water ecosystems.
The Science Behind Nitrification
Nitrification is a two-step aerobic microbial process that converts ammonia (NH3) into nitrite (NO2-) and then into nitrate (NO3-), primarily driven by specialized bacteria such as Nitrosomonas and Nitrobacter. This biochemical transformation plays a crucial role in the nitrogen cycle, influencing soil fertility and plant nutrition by making nitrogen accessible in forms usable by plants. The enzymatic reactions involved, including ammonia monooxygenase and nitrite oxidoreductase, facilitate the oxidation reactions essential for maintaining ecosystem nitrogen balance.
Steps and Microorganisms Involved in Nitrification
Nitrification involves a two-step aerobic process where ammonia-oxidizing bacteria (AOB), such as Nitrosomonas, convert ammonia (NH3) to nitrite (NO2-), followed by nitrite-oxidizing bacteria (NOB), like Nitrobacter, oxidizing nitrite to nitrate (NO3-). Denitrification, in contrast, is an anaerobic process conducted by facultative anaerobes including Pseudomonas and Paracoccus species, reducing nitrate to nitrogen gas (N2), completing the nitrogen cycle. The distinct microbial communities and oxygen conditions define the functional differences between nitrification and denitrification in soil and aquatic ecosystems.
Understanding Denitrification Processes
Denitrification is a critical biochemical process in the nitrogen cycle where nitrate (NO3-) is reduced to nitrogen gas (N2), thus removing bioavailable nitrogen from ecosystems and mitigating nitrogen pollution. This microbial-driven process occurs under anaerobic conditions, primarily facilitated by facultative anaerobic bacteria such as Paracoccus, Pseudomonas, and Bacillus species. Understanding denitrification involves studying factors like oxygen availability, organic carbon sources, and soil pH, which influence the efficiency of nitrogen gas production and control nitrogen flux in agricultural and aquatic environments.
Enzymes and Bacteria in Denitrification
Denitrification involves specialized enzymes such as nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase, which sequentially reduce nitrate (NO3-) to nitrogen gas (N2). Key bacteria responsible for denitrification include Pseudomonas, Paracoccus, and Bacillus species, which thrive in anoxic environments and contribute to nitrogen removal from soil and water. These denitrifying bacteria utilize these enzymes to drive the stepwise conversion of nitrogen compounds, playing a critical role in the nitrogen cycle.
Environmental Factors Influencing Both Processes
Nitrification and denitrification are influenced by key environmental factors such as oxygen availability, temperature, and pH. Nitrification requires aerobic conditions with sufficient oxygen levels, as chemoautotrophic bacteria oxidize ammonia to nitrate, while denitrification occurs under anoxic or low-oxygen conditions, where heterotrophic bacteria reduce nitrate to nitrogen gas. Soil moisture content and carbon source availability also critically affect both processes, with higher moisture promoting denitrification and organic carbon enhancing bacterial activity.
Importance in the Nitrogen Cycle
Nitrification and denitrification are critical processes in the nitrogen cycle that regulate soil fertility and ecosystem productivity. Nitrification converts ammonia into nitrate, making nitrogen available for plant uptake, while denitrification transforms nitrate into nitrogen gas, returning it to the atmosphere and preventing excess nitrogen accumulation. These processes maintain nitrogen balance, support plant growth, and reduce environmental impacts such as soil acidification and water pollution.
Applications in Wastewater Treatment
Nitrification and denitrification are critical processes in wastewater treatment for nitrogen removal, enhancing effluent quality and preventing eutrophication. Nitrification converts ammonia into nitrate through aerobic bacteria, while denitrification reduces nitrate to nitrogen gas under anoxic conditions, effectively eliminating nitrogen compounds. Integrating these biological processes in treatment plants improves nitrogen cycle management, meeting regulatory standards and protecting aquatic ecosystems.
Challenges and Future Perspectives
Nitrification and denitrification processes face challenges such as sensitivity to environmental conditions, including pH, temperature, and oxygen levels, which can disrupt microbial activity and nitrogen cycle efficiency. Advances in molecular techniques and bioengineering offer future perspectives for enhancing these processes through targeted microbial management and genetic modifications to improve nitrogen removal in wastewater treatment and agricultural systems. Integration of real-time monitoring and AI-driven control systems holds promise for optimizing nitrification and denitrification under variable environmental conditions.
Nitrification Infographic
 libterm.com
libterm.com