Nitrogen fixation is a crucial natural process where atmospheric nitrogen is converted into ammonia, making nitrogen available for plants to absorb and use for growth. This biological transformation primarily occurs through symbiotic bacteria in legume roots and free-living microorganisms in soil, enhancing soil fertility without synthetic fertilizers. Explore the article to understand how nitrogen fixation impacts ecosystems and your role in supporting this essential cycle.
Table of Comparison
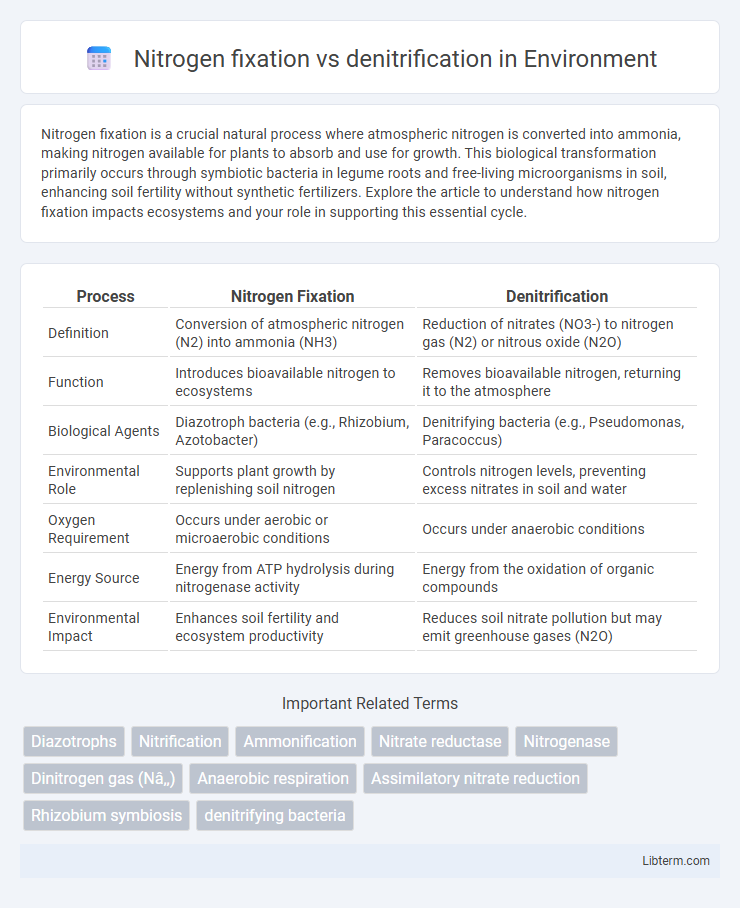
| Process | Nitrogen Fixation | Denitrification |
|---|---|---|
| Definition | Conversion of atmospheric nitrogen (N2) into ammonia (NH3) | Reduction of nitrates (NO3-) to nitrogen gas (N2) or nitrous oxide (N2O) |
| Function | Introduces bioavailable nitrogen to ecosystems | Removes bioavailable nitrogen, returning it to the atmosphere |
| Biological Agents | Diazotroph bacteria (e.g., Rhizobium, Azotobacter) | Denitrifying bacteria (e.g., Pseudomonas, Paracoccus) |
| Environmental Role | Supports plant growth by replenishing soil nitrogen | Controls nitrogen levels, preventing excess nitrates in soil and water |
| Oxygen Requirement | Occurs under aerobic or microaerobic conditions | Occurs under anaerobic conditions |
| Energy Source | Energy from ATP hydrolysis during nitrogenase activity | Energy from the oxidation of organic compounds |
| Environmental Impact | Enhances soil fertility and ecosystem productivity | Reduces soil nitrate pollution but may emit greenhouse gases (N2O) |
Introduction to Nitrogen Fixation and Denitrification
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen bioavailable for plants and microorganisms, primarily through biological processes involving diazotrophic bacteria such as Rhizobium and Azotobacter. Denitrification is a microbial process that reduces nitrate (NO3-) to gaseous nitrogen forms (N2 or N2O), returning nitrogen to the atmosphere and completing the nitrogen cycle. These complementary biochemical pathways regulate soil nitrogen levels, influencing ecosystem productivity and nitrogen availability.
The Nitrogen Cycle: An Overview
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen accessible to plants and initiating the nitrogen cycle. Denitrification reverses this process by converting nitrates (NO3-) back into atmospheric nitrogen, completing the cycle and maintaining nitrogen balance in ecosystems. These microbial processes regulate soil fertility and influence nitrogen availability in terrestrial and aquatic environments.
What is Nitrogen Fixation?
Nitrogen fixation is the biological process by which atmospheric nitrogen (N2) is converted into ammonia (NH3), making nitrogen accessible for plant use. Specialized bacteria, such as Rhizobium species found in legume root nodules, are primarily responsible for this conversion through the nitrogenase enzyme complex. This process is essential for replenishing soil nitrogen levels, supporting ecosystem productivity and agricultural sustainability.
Mechanisms and Types of Nitrogen Fixation
Nitrogen fixation involves the enzymatic conversion of atmospheric nitrogen (N2) into ammonia (NH3) by nitrogenase enzymes found in diazotrophic bacteria, including symbiotic types like Rhizobium and free-living types like Azotobacter. This biologically fixed nitrogen becomes available for plant uptake and growth, distinguishing it from denitrification, which is a microbial process whereby nitrate (NO3-) is reduced to gaseous nitrogen forms (N2 or N2O) under anaerobic conditions, returning nitrogen to the atmosphere. Types of nitrogen fixation include biological nitrogen fixation, industrial fixation via the Haber-Bosch process, and abiotic fixation through lightning, with biological fixation playing a crucial role in maintaining soil fertility.
Understanding Denitrification
Denitrification is a microbial process that converts nitrate (NO3-) into nitrogen gas (N2), effectively removing bioavailable nitrogen from soil and water. This process is crucial in the nitrogen cycle for reducing nitrogen pollution and preventing eutrophication in aquatic ecosystems. Denitrifying bacteria, such as Pseudomonas and Clostridium species, thrive in anaerobic conditions and use nitrate as an alternative electron acceptor during respiration.
Key Microorganisms Involved in Both Processes
Nitrogen fixation is primarily driven by diazotrophic bacteria such as Rhizobium species in legume root nodules and free-living Azotobacter in soil, converting atmospheric nitrogen (N2) into ammonia (NH3) for plant use. Denitrification involves anaerobic bacteria like Pseudomonas, Paracoccus, and Bacillus species that reduce nitrate (NO3-) to gaseous nitrogen forms (N2, N2O), returning nitrogen to the atmosphere. Both processes are crucial in the nitrogen cycle, influencing soil fertility and ecosystem nitrogen balance through distinct microbial pathways.
Environmental Factors Affecting Nitrogen Fixation and Denitrification
Environmental factors such as soil temperature, moisture, oxygen levels, and pH significantly influence nitrogen fixation and denitrification processes. Nitrogen fixation thrives in aerobic conditions with optimal temperatures between 25-30degC and neutral to slightly alkaline pH, while denitrification occurs under anaerobic conditions, favoring moist, low-oxygen environments with a pH around neutral. Organic carbon availability also regulates denitrification rates, making soil carbon content a critical factor in balancing nitrogen cycles.
Ecological and Agricultural Significance
Nitrogen fixation converts atmospheric nitrogen into ammonia, enriching soil fertility and supporting plant growth essential for sustainable agriculture. Denitrification reduces soil nitrate to gaseous nitrogen, mitigating nitrogen leaching and preventing groundwater contamination, crucial for maintaining ecosystem health. Both processes regulate the nitrogen cycle, balancing nutrient availability and minimizing environmental pollution in agroecosystems.
Comparison: Nitrogen Fixation vs. Denitrification
Nitrogen fixation converts atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen available for plant uptake, whereas denitrification transforms nitrates (NO3-) back into nitrogen gas (N2), removing bioavailable nitrogen from the soil. Nitrogen fixation is primarily carried out by symbiotic bacteria such as Rhizobium in legume root nodules and free-living microorganisms, while denitrification is performed by anaerobic bacteria like Pseudomonas and Clostridium under low-oxygen conditions. These processes together regulate the nitrogen cycle, balancing nitrogen input and loss in ecosystems to maintain soil fertility and reduce nitrogen pollution.
Conclusion: Balancing Nitrogen in Ecosystems
Nitrogen fixation converts atmospheric nitrogen into bioavailable forms, enriching soil and supporting plant growth, while denitrification returns nitrogen to the atmosphere, reducing bioavailable nitrogen and preventing excess accumulation. Maintaining a balance between these processes is crucial for sustaining ecosystem productivity, nutrient cycling, and minimizing environmental impacts such as eutrophication. Effective management of nitrogen dynamics enhances soil fertility and promotes ecological stability across terrestrial and aquatic systems.
Nitrogen fixation Infographic
 libterm.com
libterm.com