A chemocline is a sharp boundary within a body of water where chemical properties, such as oxygen concentration or salinity, change rapidly over a short depth interval. This layer often separates oxygen-rich surface waters from anoxic, or oxygen-depleted, deeper waters, influencing aquatic ecosystems and biogeochemical processes. Explore the rest of the article to understand how chemoclines impact marine life and water chemistry in greater detail.
Table of Comparison
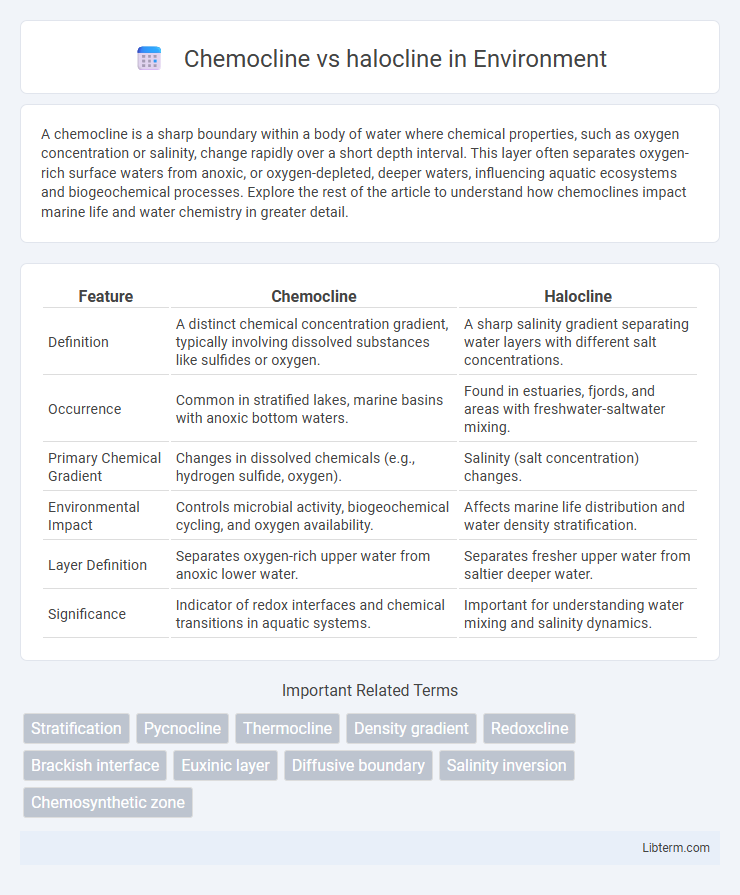
| Feature | Chemocline | Halocline |
|---|---|---|
| Definition | A distinct chemical concentration gradient, typically involving dissolved substances like sulfides or oxygen. | A sharp salinity gradient separating water layers with different salt concentrations. |
| Occurrence | Common in stratified lakes, marine basins with anoxic bottom waters. | Found in estuaries, fjords, and areas with freshwater-saltwater mixing. |
| Primary Chemical Gradient | Changes in dissolved chemicals (e.g., hydrogen sulfide, oxygen). | Salinity (salt concentration) changes. |
| Environmental Impact | Controls microbial activity, biogeochemical cycling, and oxygen availability. | Affects marine life distribution and water density stratification. |
| Layer Definition | Separates oxygen-rich upper water from anoxic lower water. | Separates fresher upper water from saltier deeper water. |
| Significance | Indicator of redox interfaces and chemical transitions in aquatic systems. | Important for understanding water mixing and salinity dynamics. |
Introduction to Chemocline and Halocline
Chemocline refers to a distinct chemical gradient in a water column, often characterized by a sharp change in dissolved substances such as sulfur or oxygen, typically found in stratified lakes or marine environments. Halocline represents a layer within a body of water where salinity changes rapidly with depth, creating a salinity gradient that significantly influences water density and mixing processes. Both chemoclines and haloclines play crucial roles in aquatic ecosystems by affecting nutrient distribution, microbial activity, and overall water chemistry.
Defining Chemocline: Key Characteristics
A chemocline is a distinct layer in a stratified water body where there is a sharp chemical gradient, typically involving differences in dissolved substances such as oxygen and sulfide levels. Unlike a halocline, which specifically refers to a rapid change in salinity, the chemocline marks the boundary between oxic and anoxic zones, often leading to unique microbial ecosystems. Key characteristics of a chemocline include steep vertical chemical gradients, reduced oxygen concentration, and the presence of sulfur compounds, which play a critical role in biogeochemical cycling.
Understanding Halocline: Basic Concepts
A halocline is a distinct layer in a body of water where salinity changes sharply with depth, significantly impacting density and water circulation. This saline gradient can create barriers to mixing between surface and deeper waters, affecting marine ecosystems and nutrient distribution. Unlike chemoclines, which involve chemical changes such as oxygen or sulfur gradients, haloclines are specifically characterized by variations in salt concentration.
Chemical Gradients in Aquatic Environments
Chemoclines represent steep chemical gradients in aquatic environments characterized primarily by changes in chemical composition, such as shifts in sulfide or oxygen concentrations, often found in stratified water bodies like meromictic lakes. Haloclines specifically denote layers where salinity gradients occur, creating barriers to mixing and influencing density-driven stratification in estuaries and marine basins. Both chemoclines and haloclines play crucial roles in controlling microbial activity, nutrient cycling, and the overall biogeochemical dynamics within aquatic ecosystems.
Salinity Layers and Halocline Formation
Chemocline and halocline represent distinct salinity layers in aquatic environments, with chemocline marking a sharp chemical gradient often caused by differences in salinity, oxygen, or other dissolved substances. Halocline specifically refers to the salinity-driven gradient where rapid changes in salt concentration occur, typically due to freshwater mixing with more saline water, leading to stratification. Halocline formation results from processes such as river inflow, evaporation, or seawater intrusion, creating distinct layers that impact water density and marine ecosystem dynamics.
Ecological Implications of Chemoclines
Chemoclines, characterized by sharp chemical gradients typically involving sulfide and oxygen, create unique anoxic habitats crucial for specialized microbial communities like sulfur-oxidizing bacteria, significantly influencing biogeochemical cycles. Unlike haloclines that primarily involve salinity gradients affecting water density and marine organism distribution, chemoclines sustain stratified ecosystems where redox processes maintain nutrient availability and support complex food webs. The ecological implications of chemoclines extend to carbon cycling, habitat formation for extremophiles, and the regulation of greenhouse gases such as methane in stratified water bodies.
Differences Between Chemocline and Halocline
A chemocline is a distinct chemical gradient in a body of water, often marked by a sharp change in dissolved chemical substances such as hydrogen sulfide, while a halocline specifically refers to a layer where salinity changes rapidly. Chemoclines are commonly found in stratified lakes with anoxic bottom waters rich in sulfur compounds, whereas haloclines occur in estuaries or marine environments where fresh and saltwater mix. The key difference lies in the nature of the chemical gradient: chemoclines focus on chemical substances affecting redox conditions, and haloclines concentrate on salinity variations.
Examples of Chemocline vs Halocline in Nature
Chemoclines occur in environments such as meromictic lakes like Lake Cadagno in Switzerland, where a distinct chemical gradient separates oxygen-rich surface waters from anoxic, sulfide-rich bottom waters. Haloclines are commonly observed in estuarine zones like the Baltic Sea, where a sharp salinity gradient exists between freshwater inflows and saline ocean water. These natural examples highlight chemoclines defined by chemical composition shifts, primarily redox conditions, while haloclines are characterized by abrupt salinity changes affecting water density stratification.
Methods for Studying Water Column Stratification
Methods for studying water column stratification in chemoclines involve analyzing chemical gradients, particularly sharp redox transitions where oxygen-rich and anoxic water layers meet, using in situ voltammetry and nutrient profiling. Haloclines are examined through salinity measurements using conductivity sensors, hydrocasts, and CTD (Conductivity, Temperature, Depth) instruments to detect abrupt changes in salinity levels between water masses. Combining these approaches with oxygen and sulfide microelectrodes provides detailed vertical profiles essential for understanding biogeochemical processes in stratified aquatic systems.
Importance of Clines in Aquatic Ecosystem Dynamics
Chemoclines and haloclines represent critical density gradients in aquatic ecosystems, with chemoclines arising from sharp chemical composition differences, such as oxygen or sulfide levels, and haloclines from abrupt salinity changes. These clines create distinct microhabitats that regulate nutrient cycling, microbial activity, and biogeochemical processes essential for maintaining ecosystem stability and productivity. The stratification imposed by chemoclines and haloclines influences oxygen distribution and species diversity, directly affecting food web interactions and overall aquatic health.
Chemocline Infographic
 libterm.com
libterm.com