Radiocarbon dating measures the decay of carbon-14 isotopes in organic materials to determine their age, providing accurate estimates for artifacts up to 50,000 years old. This method is widely used in archaeology, geology, and environmental science to trace historical timelines and understand past climates. Discover how radiocarbon dating can unlock the secrets of ancient objects in the rest of the article.
Table of Comparison
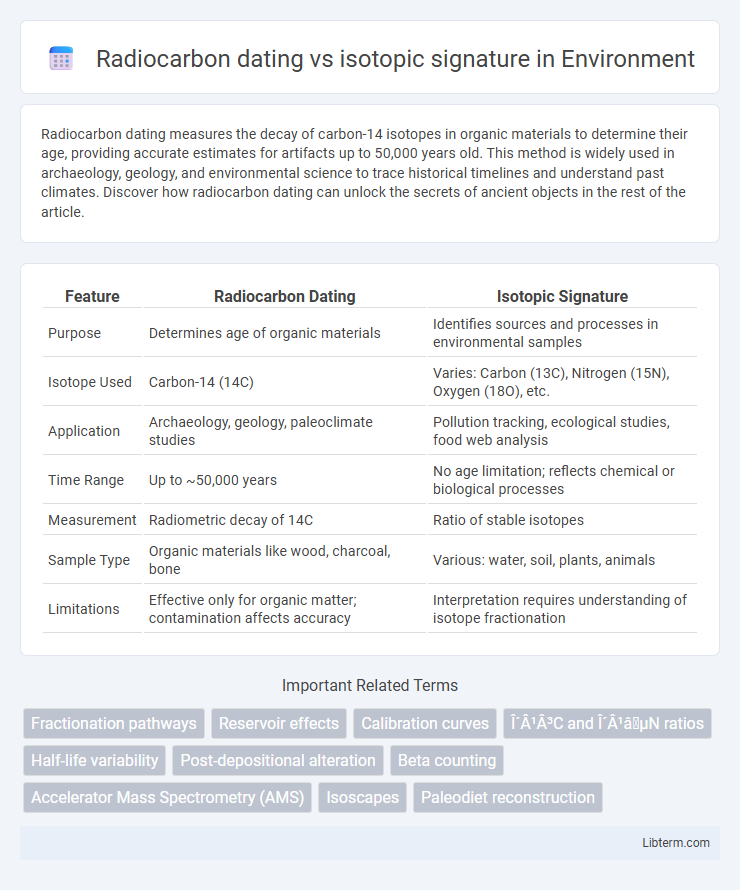
| Feature | Radiocarbon Dating | Isotopic Signature |
|---|---|---|
| Purpose | Determines age of organic materials | Identifies sources and processes in environmental samples |
| Isotope Used | Carbon-14 (14C) | Varies: Carbon (13C), Nitrogen (15N), Oxygen (18O), etc. |
| Application | Archaeology, geology, paleoclimate studies | Pollution tracking, ecological studies, food web analysis |
| Time Range | Up to ~50,000 years | No age limitation; reflects chemical or biological processes |
| Measurement | Radiometric decay of 14C | Ratio of stable isotopes |
| Sample Type | Organic materials like wood, charcoal, bone | Various: water, soil, plants, animals |
| Limitations | Effective only for organic matter; contamination affects accuracy | Interpretation requires understanding of isotope fractionation |
Introduction to Radiocarbon Dating and Isotopic Signature
Radiocarbon dating measures the decay of carbon-14 isotopes to determine the age of organic materials up to about 50,000 years old, providing a reliable chronological framework for archaeological and geological samples. Isotopic signature analysis examines the ratios of stable isotopes, such as carbon-13 to carbon-12, to reveal information about the origin, diet, and environmental conditions affecting a sample. These methodologies complement each other by offering both temporal dating and detailed compositional insights critical for reconstructing historical and ecological contexts.
Principles Behind Radiocarbon Dating
Radiocarbon dating relies on measuring the decay of carbon-14 isotopes in organic materials to estimate age, exploiting the known half-life of carbon-14, approximately 5,730 years. This method calculates the time elapsed since the death of an organism by comparing the ratio of carbon-14 to stable carbon isotopes, such as carbon-12. Unlike isotopic signature analysis, which identifies the origins and pathways of elements based on various isotope ratios, radiocarbon dating specifically uses carbon-14 decay as a precise chronological tool for dating archaeological and geological samples up to about 50,000 years old.
Fundamentals of Isotopic Signatures
Isotopic signatures rely on the ratio of stable isotopes within a material, reflecting environmental and biological processes rather than age alone, unlike radiocarbon dating which measures the decay of carbon-14 to estimate age. The fundamental principle involves measuring variations in isotopes such as carbon-13, oxygen-18, or nitrogen-15, which provide insights into origins, diet, and climatic conditions. Radiocarbon dating is limited to organic materials up to about 50,000 years, while isotopic signatures offer broader applications in archaeology, ecology, and geology by tracing sources and environmental interactions.
Differences in Methodology
Radiocarbon dating measures the decay of carbon-14 isotopes in organic materials to determine age, relying on radioactive decay rates as a temporal marker. Isotopic signature analysis examines stable isotope ratios, such as carbon-13/carbon-12 or oxygen-18/oxygen-16, to infer environmental conditions and sources rather than dating age. While radiocarbon dating provides chronological information through absolute dating, isotopic signature focuses on qualitative insights into biological, geological, or climatic processes through comparative isotope ratios.
Applications in Archaeology and Geology
Radiocarbon dating provides precise age estimates for organic archaeological artifacts up to about 50,000 years old, essential in constructing human history timelines and understanding cultural developments. Isotopic signature analysis extends beyond dating by tracing geological processes, climate changes, and migration patterns through stable isotope ratios in minerals and fossils, offering insights into past environmental conditions. Combining both methods enhances chronological frameworks and environmental reconstructions in archaeology and geology, deepening our interpretation of earth's historical and prehistorical events.
Strengths and Limitations of Radiocarbon Dating
Radiocarbon dating excels in determining the age of organic materials up to around 50,000 years by measuring the decay of carbon-14 isotopes, providing precise chronological insights for archaeology and geology. However, its limitations include reduced accuracy for samples older than this range due to diminishing carbon-14 levels and potential contamination that can skew results. Isotopic signature analysis complements radiocarbon dating by offering a broader perspective on environmental conditions and material origins but does not directly provide chronological data.
Strengths and Limitations of Isotopic Signature Analysis
Isotopic signature analysis excels in tracing the geographic origin and environmental history of samples, offering insights into climatic conditions and dietary patterns that radiocarbon dating cannot provide. However, its limitations include sensitivity to post-depositional alterations and the requirement for well-preserved organic material to yield accurate isotopic ratios. Unlike radiocarbon dating, which reliably determines chronological age up to 50,000 years, isotopic analysis provides contextual data rather than precise dating, making it complementary but not a substitute for radiocarbon methods.
Comparative Accuracy and Reliability
Radiocarbon dating offers high accuracy for dating organic remains up to 50,000 years old by measuring the decay of carbon-14 isotopes, but its reliability diminishes with older samples or contamination. Isotopic signature analysis provides a broader scope by examining stable isotopes like carbon-13 and nitrogen-15 to trace environmental and dietary patterns, offering consistent reliability across varied sample types but less direct age determination. Combining both methods enhances chronological precision and contextual understanding, improving overall reliability in archaeological and geological studies.
Recent Advances and Innovations
Recent advances in radiocarbon dating leverage improved accelerator mass spectrometry (AMS) techniques, enhancing precision and allowing for more accurate age determinations in archaeological and environmental samples. Innovations in isotopic signature analysis integrate multi-isotope frameworks, combining radiocarbon with stable isotopes such as carbon-13 and nitrogen-15 to refine source attribution and paleoclimate reconstructions. Emerging methodologies employ compound-specific radiocarbon dating, targeting individual organic molecules to overcome contamination challenges and provide more detailed chronological insights.
Choosing the Right Technique for Scientific Research
Radiocarbon dating is ideal for determining the age of organic materials up to 50,000 years old by measuring the decay of carbon-14 isotopes, providing precise chronological data for archaeological and geological research. Isotopic signature analysis offers insights into the environmental and biological processes affecting samples by examining stable isotopes like carbon-13, oxygen-18, or nitrogen-15, useful for tracing origins and dietary patterns. Selecting the appropriate technique depends on research goals: radiocarbon dating for age estimation and isotopic signatures for ecological or geochemical interpretations.
Radiocarbon dating Infographic
 libterm.com
libterm.com