Pharmaceuticals play a crucial role in improving health outcomes by developing medications that treat, prevent, and manage various diseases. Advances in drug research and development have led to innovative therapies that enhance patient quality of life and extend lifespan. Discover how these groundbreaking innovations in pharmaceuticals can impact your health by reading the full article.
Table of Comparison
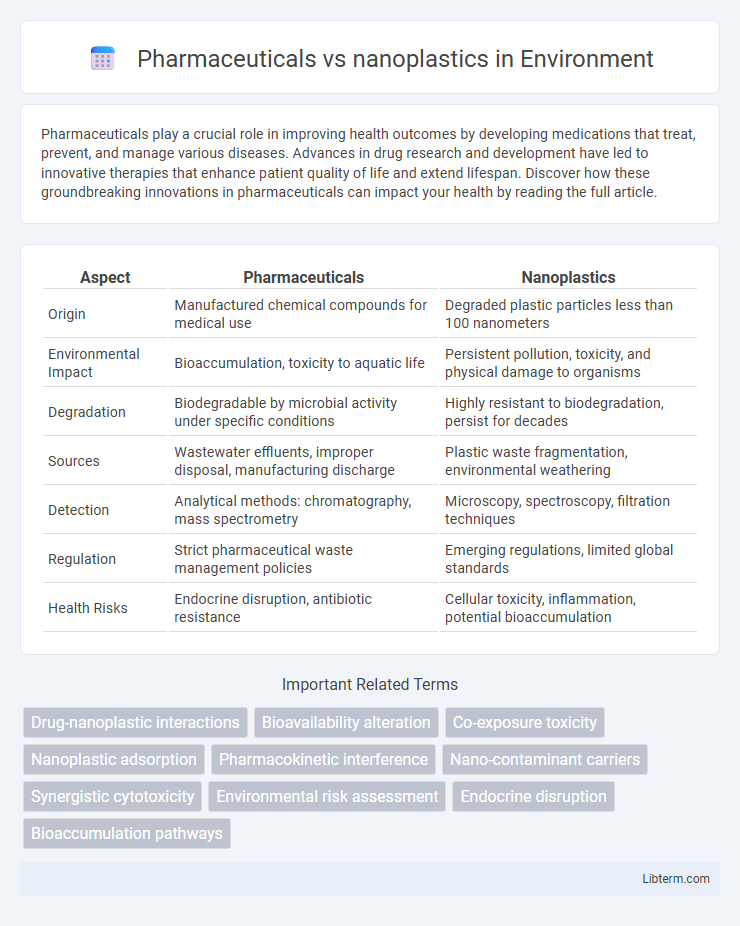
| Aspect | Pharmaceuticals | Nanoplastics |
|---|---|---|
| Origin | Manufactured chemical compounds for medical use | Degraded plastic particles less than 100 nanometers |
| Environmental Impact | Bioaccumulation, toxicity to aquatic life | Persistent pollution, toxicity, and physical damage to organisms |
| Degradation | Biodegradable by microbial activity under specific conditions | Highly resistant to biodegradation, persist for decades |
| Sources | Wastewater effluents, improper disposal, manufacturing discharge | Plastic waste fragmentation, environmental weathering |
| Detection | Analytical methods: chromatography, mass spectrometry | Microscopy, spectroscopy, filtration techniques |
| Regulation | Strict pharmaceutical waste management policies | Emerging regulations, limited global standards |
| Health Risks | Endocrine disruption, antibiotic resistance | Cellular toxicity, inflammation, potential bioaccumulation |
Introduction: Pharmaceuticals and Nanoplastics Defined
Pharmaceuticals encompass synthetic or natural chemical substances designed for diagnosing, treating, or preventing diseases in humans and animals. Nanoplastics refer to plastic particles smaller than 100 nanometers, arising from the degradation of larger plastic debris and known for their potential environmental and biological impacts. Understanding the distinct properties and behaviors of pharmaceuticals and nanoplastics is crucial for assessing their respective risks and interactions within ecosystems and human health.
Sources and Pathways into the Environment
Pharmaceuticals enter the environment primarily through human excretion and improper disposal, leaching from wastewater treatment plants into surface water and soil. Nanoplastics originate from the breakdown of larger plastic debris, release from commercial products, and industrial processes, infiltrating aquatic and terrestrial ecosystems via runoff and air deposition. Both contaminants persist and accumulate, posing emerging risks to environmental and human health through complex transport pathways.
Chemical Composition and Structural Differences
Pharmaceuticals are composed of carefully engineered active pharmaceutical ingredients (APIs) with specific molecular structures designed for targeted therapeutic effects, often featuring complex organic compounds such as alkaloids, steroids, or peptides. Nanoplastics consist primarily of fragmented polymers like polyethylene, polypropylene, or polystyrene, characterized by irregular shapes and amorphous or semi-crystalline structures resulting from environmental degradation processes. The distinct chemical compositions and structural makeups influence their interaction with biological systems, where pharmaceuticals exhibit predictable bioactivity, and nanoplastics pose challenges due to their variable surface chemistry and size-dependent behavior.
Environmental Persistence and Degradation
Pharmaceuticals often exhibit variable environmental persistence due to their complex chemical structures, with some compounds degrading slowly and accumulating in water bodies. Nanoplastics demonstrate extreme resistance to natural degradation processes, persisting for decades or longer in diverse environmental matrices like soil, sediments, and oceans. The slow breakdown of both substances poses significant risks to ecosystems, necessitating advanced remediation strategies tailored to their distinct degradation profiles.
Bioaccumulation and Food Chain Impacts
Pharmaceuticals and nanoplastics both pose significant risks through bioaccumulation in aquatic organisms, with pharmaceuticals often disrupting endocrine and metabolic processes, while nanoplastics can cause physical damage and transport toxic contaminants. Bioaccumulation of these substances leads to biomagnification across trophic levels, ultimately threatening biodiversity and human health through contaminated seafood. Understanding their distinct modes of action is critical for assessing cumulative impacts on food chain stability and ecosystem services.
Human Health Risks and Exposure Routes
Pharmaceuticals entering aquatic environments pose significant human health risks through direct consumption of contaminated water and bioaccumulation in seafood, leading to antibiotic resistance and endocrine disruption. Nanoplastics, pervasive in air, water, and food, penetrate biological barriers causing oxidative stress, inflammation, and potential neurotoxicity in humans. Both contaminants exploit multiple exposure routes including ingestion, inhalation, and dermal contact, intensifying cumulative toxicological effects on public health.
Detection, Monitoring, and Analytical Techniques
Advanced detection and monitoring of pharmaceuticals and nanoplastics rely on cutting-edge analytical techniques such as liquid chromatography-mass spectrometry (LC-MS) and nanoparticle tracking analysis (NTA). These methods enable precise quantification and characterization of trace contaminants in environmental and biological samples, facilitating accurate assessment of their distribution and potential impacts. Emerging sensors and spectroscopic tools further enhance sensitivity and selectivity, driving improved environmental risk evaluation and regulatory compliance.
Regulatory Frameworks and Global Guidelines
Regulatory frameworks for pharmaceuticals and nanoplastics diverge significantly, with pharmaceuticals subject to stringent approval processes by agencies such as the FDA and EMA, enforcing safety, efficacy, and environmental impact assessments. Nanoplastics, being an emerging contaminant, face a lack of comprehensive global regulatory guidelines, though organizations like the UN Environment Programme and OECD are actively developing standards to monitor and mitigate environmental risks. International cooperation and harmonization of policies remain critical to address the unique challenges posed by nanoplastics while maintaining robust oversight of pharmaceutical pollutants.
Mitigation Strategies and Waste Management
Mitigation strategies for pharmaceuticals and nanoplastics involve advanced filtration technologies such as membrane bioreactors and activated carbon adsorption to remove contaminants from wastewater effectively. Waste management practices prioritize source reduction by promoting proper disposal of medications and minimizing plastic use, complemented by enhanced recycling systems targeting nanoplastic particles. Emerging solutions emphasize integrated treatment processes combining chemical, biological, and physical methods to ensure comprehensive removal and environmental safety.
Future Perspectives and Research Gaps
Future research on pharmaceuticals versus nanoplastics must prioritize advanced detection methods and toxicological assessments to understand their combined environmental and health impacts. Emerging nanotechnology tools could enable precise monitoring of nanoparticle interactions with pharmaceutical compounds, revealing synergistic effects and biodegradation pathways. Addressing regulatory gaps and developing standardized protocols are essential for managing risks associated with the coexistence of pharmaceuticals and nanoplastics in ecosystems.
Pharmaceuticals Infographic
 libterm.com
libterm.com