Metal-rich mine drainage contains high concentrations of toxic metals such as iron, copper, and zinc that can severely contaminate water sources and harm aquatic ecosystems. Effective treatment methods focus on neutralizing acidity and precipitating metals to prevent environmental damage. Explore this article to discover how innovative solutions can protect your local waterways from metal-rich mine drainage.
Table of Comparison
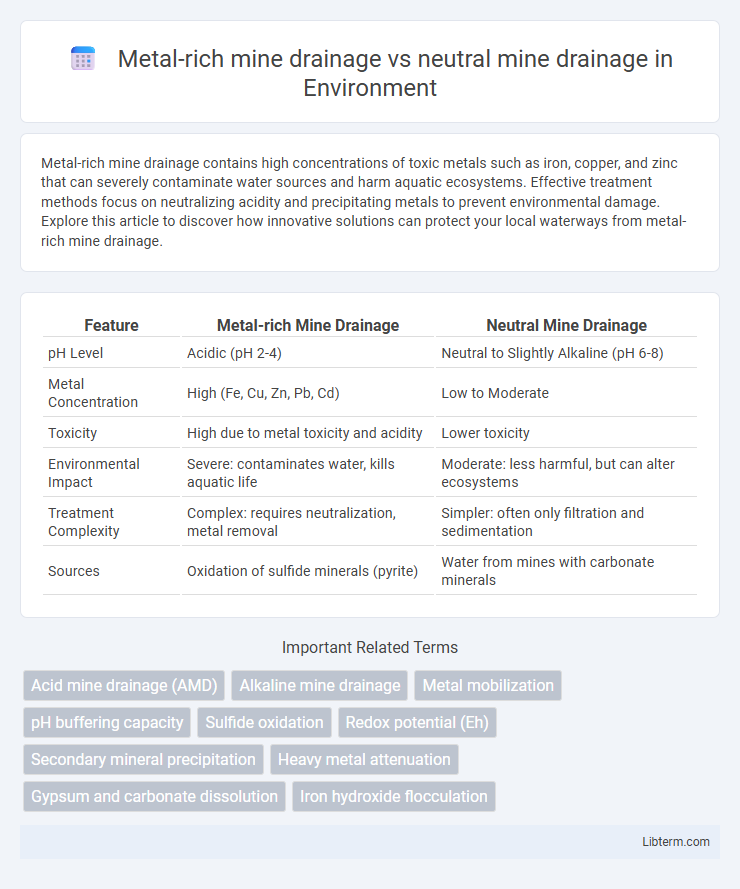
| Feature | Metal-rich Mine Drainage | Neutral Mine Drainage |
|---|---|---|
| pH Level | Acidic (pH 2-4) | Neutral to Slightly Alkaline (pH 6-8) |
| Metal Concentration | High (Fe, Cu, Zn, Pb, Cd) | Low to Moderate |
| Toxicity | High due to metal toxicity and acidity | Lower toxicity |
| Environmental Impact | Severe: contaminates water, kills aquatic life | Moderate: less harmful, but can alter ecosystems |
| Treatment Complexity | Complex: requires neutralization, metal removal | Simpler: often only filtration and sedimentation |
| Sources | Oxidation of sulfide minerals (pyrite) | Water from mines with carbonate minerals |
Introduction to Metal-rich and Neutral Mine Drainage
Metal-rich mine drainage contains high concentrations of dissolved metals such as iron, copper, zinc, and lead, often resulting from the oxidation of sulfide minerals in exposed mine wastes and tailings. Neutral mine drainage, characterized by near-neutral pH levels, typically exhibits lower metal solubility but may still carry elevated levels of dissolved metals due to complex geochemical interactions. Understanding the geochemical differences between metal-rich and neutral mine drainage is critical for designing effective remediation strategies and minimizing environmental impacts on surrounding ecosystems.
Formation Processes of Mine Drainage Types
Metal-rich mine drainage forms primarily through the oxidation of sulfide minerals such as pyrite and chalcopyrite, releasing high concentrations of metals and acidity into surrounding waters. Neutral mine drainage occurs when carbonate minerals, like calcite, buffer acidity released from sulfide oxidation, resulting in near-neutral pH and lower metal solubility. The interaction between sulfide oxidation rates, mineral buffering capacity, and environmental conditions dictates the specific formation process and chemical characteristics of each mine drainage type.
Key Chemical Characteristics
Metal-rich mine drainage typically exhibits low pH levels, often below 4, with high concentrations of dissolved metals such as iron, copper, zinc, and lead, resulting from the oxidation of sulfide minerals. Neutral mine drainage maintains a near-neutral pH, usually between 6 and 8, with lower metal ion concentrations due to the buffering capacity of carbonate minerals present in the host rock. The key chemical difference lies in acidity and metal solubility, where metal-rich drainage promotes metal mobility and toxicity, whereas neutral drainage minimizes metal dissolution and environmental impact.
Major Environmental Impacts
Metal-rich mine drainage, often characterized by low pH and high concentrations of heavy metals such as iron, copper, and zinc, causes severe acidification of aquatic ecosystems and bioaccumulation of toxic metals in flora and fauna. Neutral mine drainage, with near-neutral pH and elevated sulfate and metal content, primarily leads to sediment contamination and alters water chemistry, impacting benthic habitats and aquatic biodiversity. Both drainage types contribute to long-term soil degradation and groundwater contamination, posing significant challenges for ecosystem restoration and water quality management.
Metal Mobility and Bioavailability
Metal-rich mine drainage exhibits higher metal mobility due to acidic conditions that increase metal solubility and bioavailability, leading to greater ecological toxicity. Neutral mine drainage maintains near-neutral pH levels, which promote metal precipitation and reduced metal mobility, thereby decreasing bioavailability and environmental impact. The contrasting chemical environments in these drainage types significantly affect the transport, bioaccumulation, and toxicity of metals such as iron, copper, lead, and zinc.
Affected Ecosystems and Biodiversity
Metal-rich mine drainage often results in highly acidic waters with elevated concentrations of heavy metals such as arsenic, lead, and mercury, severely harming aquatic ecosystems by disrupting physiological processes and reducing biodiversity. In contrast, neutral mine drainage maintains near-neutral pH levels but still contains dissolved metals that can accumulate in sediments and bioaccumulate in aquatic organisms, causing sub-lethal toxicity and altering species composition. Both drainage types degrade ecosystem function, but metal-rich drainage typically causes more immediate and severe declines in sensitive species, while neutral drainage poses chronic risks through long-term metal exposure.
Remediation Challenges and Strategies
Metal-rich mine drainage often contains high concentrations of heavy metals like iron, copper, and zinc, posing significant remediation challenges due to metal toxicity and complex precipitation reactions. Neutral mine drainage, typically having near-neutral pH but elevated sulfate and metal levels, requires strategies focusing on metal removal through biological sulfate reduction or chemical precipitation. Effective remediation for both types demands site-specific approaches combining active and passive treatments such as constructed wetlands, lime neutralization, and bioreactors to optimize metal removal and mitigate environmental impact.
Monitoring and Assessment Methods
Metal-rich mine drainage requires continuous monitoring of heavy metal concentrations using techniques like atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) to detect toxic elements such as cadmium, lead, and arsenic. Neutral mine drainage, often characterized by near-neutral pH and lower metal solubility, is typically assessed through periodic sampling and analysis of parameters like pH, electrical conductivity, and sulfate levels to evaluate potential impacts on downstream ecosystems. Both types benefit from deploying automated sensors and remote sensing technologies to provide real-time data for effective environmental risk assessment and remediation planning.
Regulatory Standards and Compliance
Metal-rich mine drainage typically requires stringent regulatory standards due to its high concentrations of heavy metals like arsenic, lead, and cadmium, necessitating advanced treatment technologies to meet environmental compliance limits set by agencies such as the EPA and EU Water Framework Directive. Neutral mine drainage, characterized by near-neutral pH and lower metal loads, often falls under less rigorous regulatory thresholds but still demands regular monitoring to prevent ecological harm and ensure compliance with water quality standards. Both drainage types must adhere to site-specific permits and discharge limits, with compliance enforced through comprehensive reporting, remediation efforts, and consistent water quality assessments.
Future Trends in Mine Drainage Management
Future trends in mine drainage management emphasize advanced treatment technologies tailored to metal-rich mine drainage, including bio-remediation with metal-tolerant microorganisms and innovative passive treatment systems that enhance metal recovery. Neutral mine drainage management increasingly incorporates real-time monitoring and predictive modeling to optimize water quality control and reduce treatment costs. Combined approaches integrating circular economy principles promote resource recovery and sustainable closure practices for both metal-rich and neutral mine drainage sites.
Metal-rich mine drainage Infographic
 libterm.com
libterm.com