Nitrification is a crucial process in the nitrogen cycle where ammonia is converted into nitrites and then nitrates by specialized bacteria, enhancing soil fertility and plant growth. Understanding this process helps optimize agricultural practices and improve environmental management by reducing harmful nitrogen runoff. Explore the article to learn how nitrification impacts your ecosystem and ways to support this essential natural process.
Table of Comparison
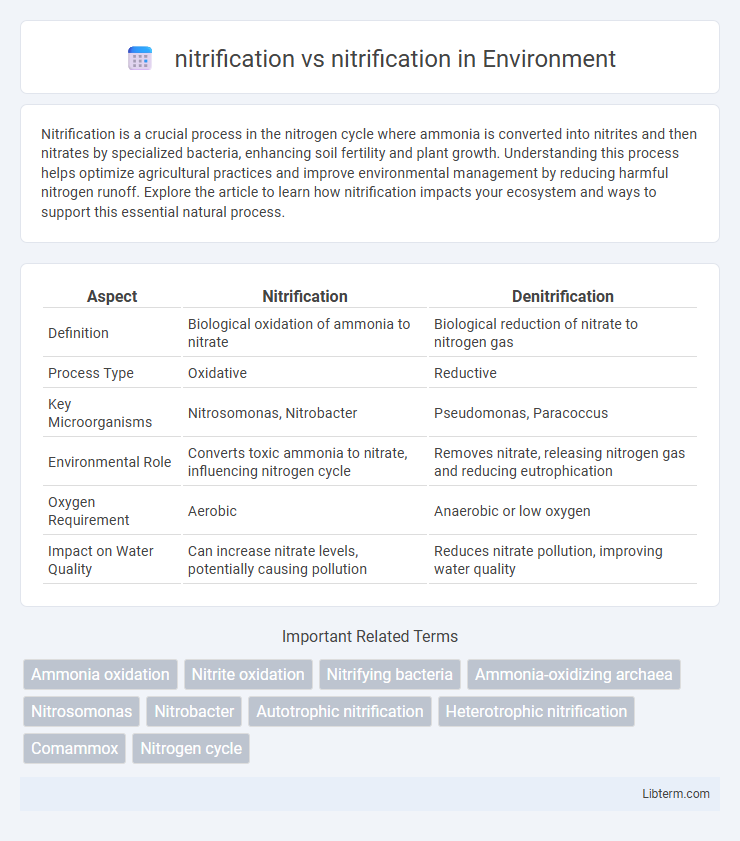
| Aspect | Nitrification | Denitrification |
|---|---|---|
| Definition | Biological oxidation of ammonia to nitrate | Biological reduction of nitrate to nitrogen gas |
| Process Type | Oxidative | Reductive |
| Key Microorganisms | Nitrosomonas, Nitrobacter | Pseudomonas, Paracoccus |
| Environmental Role | Converts toxic ammonia to nitrate, influencing nitrogen cycle | Removes nitrate, releasing nitrogen gas and reducing eutrophication |
| Oxygen Requirement | Aerobic | Anaerobic or low oxygen |
| Impact on Water Quality | Can increase nitrate levels, potentially causing pollution | Reduces nitrate pollution, improving water quality |
Introduction to Nitrification
Nitrification is a critical microbial process in the nitrogen cycle where ammonia (NH3) is oxidized to nitrite (NO2-) and then to nitrate (NO3-) by specialized bacteria such as Nitrosomonas and Nitrobacter. This biochemical conversion enhances soil fertility and plays a vital role in wastewater treatment by reducing toxic ammonia levels. Understanding the mechanisms and environmental factors affecting nitrification is essential for optimizing agricultural productivity and maintaining ecosystem health.
Overview of the Nitrification Process
Nitrification is a crucial microbial process in the nitrogen cycle where ammonia (NH3) is oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB) or archaea (AOA), followed by the conversion of nitrite to nitrate (NO3-) by nitrite-oxidizing bacteria (NOB). This two-step aerobic process increases nitrate availability in soils, essential for plant uptake and ecosystem productivity. The rate and efficiency of nitrification are influenced by factors such as soil pH, temperature, oxygen availability, and organic matter content.
Key Stages in Nitrification
Nitrification involves two key stages: the oxidation of ammonia (NH3) to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB) or archaea (AOA), followed by the conversion of nitrite to nitrate (NO3-) by nitrite-oxidizing bacteria (NOB). The first stage is critical for removing toxic ammonia from environments, which is then rapidly transformed into nitrate, a form readily absorbed by plants. Understanding these stages enhances wastewater treatment processes and soil nitrogen cycling efficiency.
Microbial Players in Nitrification
Nitrification is a two-step microbial process involving ammonia-oxidizing bacteria (AOB) and archaea (AOA) that convert ammonia (NH3) into nitrite (NO2-), followed by nitrite-oxidizing bacteria (NOB) that oxidize nitrite into nitrate (NO3-). Key AOB genera include Nitrosomonas and Nitrosospira, while prominent NOB genera comprise Nitrobacter and Nitrospira, with archaea like Nitrososphaera playing significant roles in ammonia oxidation in various environments. The efficiency and environmental adaptability of these microbes are critical for nitrogen cycling in soils, aquatic systems, and wastewater treatment processes.
Environmental Factors Affecting Nitrification
Environmental factors significantly influence nitrification, including temperature, pH, oxygen availability, and ammonia concentration. Optimal nitrification rates occur in neutral to slightly alkaline pH (6.5-8.5) and temperatures between 20-30degC, while low oxygen levels and extreme pH inhibit microbial activity of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria. Variations in soil moisture and the presence of inhibitors such as heavy metals or pesticides also impact the efficiency of nitrification processes in ecosystems.
Importance of Nitrification in Nitrogen Cycle
Nitrification plays a crucial role in the nitrogen cycle by converting ammonia into nitrites and then nitrates, which are essential forms of nitrogen readily absorbed by plants. This biological oxidation process, primarily carried out by nitrifying bacteria such as Nitrosomonas and Nitrobacter, ensures the transformation of toxic ammonia into nitrate, facilitating nutrient availability and ecosystem productivity. Efficient nitrification supports soil fertility, promotes plant growth, and sustains agricultural productivity by maintaining the nitrogen balance in terrestrial environments.
Applications of Nitrification in Wastewater Treatment
Nitrification plays a crucial role in wastewater treatment by converting harmful ammonia into less toxic nitrate, thus preventing ammonia toxicity in aquatic ecosystems. This biological process, driven by autotrophic bacteria such as Nitrosomonas and Nitrobacter, enhances nitrogen removal and protects water quality in municipal and industrial wastewater facilities. Optimizing parameters like oxygen levels and pH can significantly improve nitrification efficiency, leading to more sustainable and compliant effluent discharge.
Challenges and Limitations of Nitrification
Nitrification faces challenges such as sensitivity to pH fluctuations, temperature variations, and inhibitory substances like heavy metals or high ammonia concentrations, which can significantly reduce microbial activity. The process is limited by the slow growth rate of nitrifying bacteria, resulting in longer treatment times and vulnerability to environmental stressors. Furthermore, incomplete nitrification can lead to the accumulation of toxic intermediates like nitrite, posing risks to aquatic ecosystems and water treatment systems.
Nitrification vs. Denitrification: A Comparative Analysis
Nitrification is the aerobic biological oxidation of ammonia (NH3) to nitrate (NO3-) through intermediate nitrite (NO2-) by nitrifying bacteria such as Nitrosomonas and Nitrobacter, playing a crucial role in the nitrogen cycle by converting toxic ammonia to less harmful nitrate. Denitrification, in contrast, is an anaerobic process carried out by bacteria like Pseudomonas and Clostridium that reduces nitrate to nitrogen gas (N2), effectively removing bioavailable nitrogen from ecosystems and mitigating nitrate pollution. Both processes regulate nitrogen availability but operate under different environmental conditions and have opposite impacts on nitrogen retention and loss in soil and aquatic systems.
Future Perspectives in Nitrification Research
Future perspectives in nitrification research emphasize advancements in microbial ecology and genomics to uncover novel nitrifying organisms and pathways, improving nitrogen cycling efficiency. Innovative strategies in biotechnological applications aim to optimize nitrification processes for sustainable agriculture and wastewater treatment, reducing environmental impacts like greenhouse gas emissions. Continued exploration of enzyme mechanisms and regulatory networks holds potential for developing targeted interventions to enhance nitrification under diverse environmental conditions.
nitrification Infographic
 libterm.com
libterm.com