Hydrolysis is a chemical process where water molecules break down complex compounds into simpler ones by splitting chemical bonds. This reaction is essential in various biological and industrial applications, such as digestion and the production of biofuels. Discover how hydrolysis plays a vital role in everyday life and scientific advancements by reading the full article.
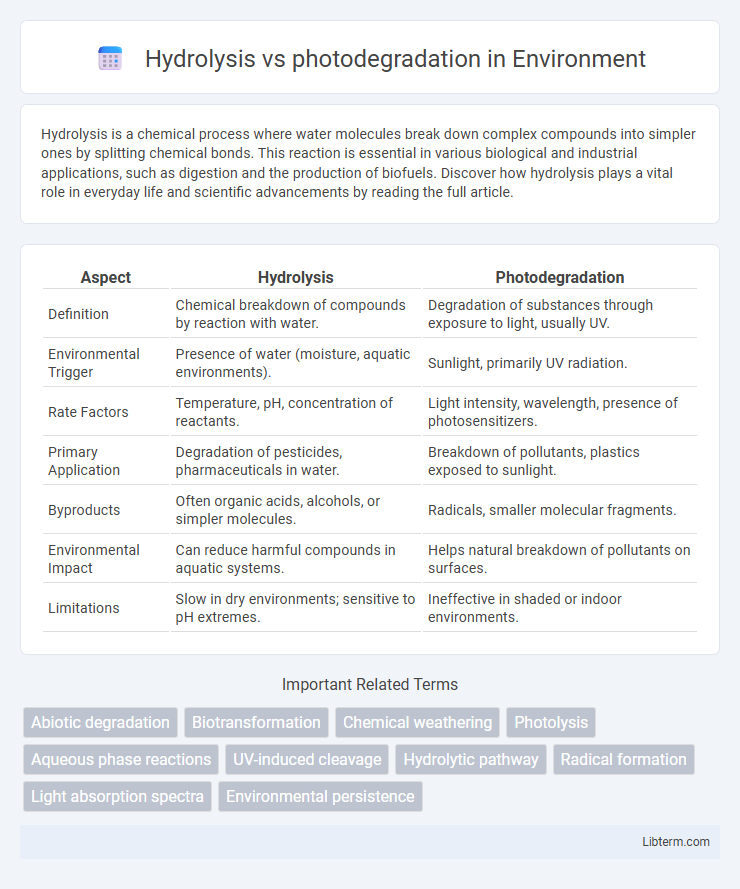
Table of Comparison
| Aspect | Hydrolysis | Photodegradation |
|---|---|---|
| Definition | Chemical breakdown of compounds by reaction with water. | Degradation of substances through exposure to light, usually UV. |
| Environmental Trigger | Presence of water (moisture, aquatic environments). | Sunlight, primarily UV radiation. |
| Rate Factors | Temperature, pH, concentration of reactants. | Light intensity, wavelength, presence of photosensitizers. |
| Primary Application | Degradation of pesticides, pharmaceuticals in water. | Breakdown of pollutants, plastics exposed to sunlight. |
| Byproducts | Often organic acids, alcohols, or simpler molecules. | Radicals, smaller molecular fragments. |
| Environmental Impact | Can reduce harmful compounds in aquatic systems. | Helps natural breakdown of pollutants on surfaces. |
| Limitations | Slow in dry environments; sensitive to pH extremes. | Ineffective in shaded or indoor environments. |
Introduction to Hydrolysis and Photodegradation
Hydrolysis is a chemical process involving the breakdown of compounds through reaction with water, often catalyzed by acids, bases, or enzymes. Photodegradation refers to the decomposition of chemical substances caused by exposure to light, particularly ultraviolet (UV) radiation, leading to structural changes in molecules. Both processes are critical in environmental chemistry for the natural degradation and transformation of pollutants and organic materials.
Definitions and Key Concepts
Hydrolysis is a chemical process in which water molecules break down compounds, often resulting in the decomposition of pollutants or organic materials in aqueous environments. Photodegradation involves the breakdown of chemical substances under exposure to light, particularly ultraviolet (UV) radiation, leading to molecular changes or complete mineralization. Both processes are crucial in environmental chemistry for the natural attenuation of contaminants, with hydrolysis primarily driven by water and photodegradation by light energy.
Mechanisms of Hydrolysis
Hydrolysis involves the chemical breakdown of a compound due to reaction with water, often catalyzed by acids, bases, or enzymes, leading to cleavage of bonds such as esters, amides, or anhydrides. This mechanism typically results in the formation of smaller, more hydrophilic molecules, increasing the compound's solubility and facilitating further degradation or biotransformation. In contrast, photodegradation uses light energy, primarily ultraviolet radiation, to break chemical bonds through photochemical reactions, producing free radicals or excited states that drive molecular breakdown.
Mechanisms of Photodegradation
Photodegradation involves the breakdown of chemical compounds through the absorption of light, primarily ultraviolet (UV) radiation, which excites molecules leading to bond cleavage and formation of reactive free radicals. This mechanism includes direct photolysis, where the compound absorbs photons causing molecular fragmentation, and indirect photolysis, where light-activated photosensitizers produce reactive species like singlet oxygen or hydroxyl radicals that attack target molecules. Hydrolysis, in contrast, relies on chemical reaction with water molecules to cleave bonds, without the involvement of light-induced energy or radical intermediates.
Factors Influencing Hydrolysis
Hydrolysis rates are influenced by several factors including pH, temperature, and the chemical structure of the compound, particularly the presence of hydrolyzable functional groups like esters, amides, and anhydrides. The reaction typically accelerates in either strongly acidic or alkaline conditions, with temperature increases enhancing molecular collisions and reaction kinetics. Unlike photodegradation, which depends on light intensity and wavelength, hydrolysis strictly relies on aqueous conditions and the availability of water molecules to break chemical bonds.
Factors Influencing Photodegradation
Photodegradation is influenced primarily by environmental factors such as light intensity, wavelength, and the presence of photosensitizers, which facilitate the breakdown of chemical compounds. Temperature and oxygen concentration also play significant roles by affecting the rate of photo-induced reactions and the formation of reactive oxygen species. Compared to hydrolysis, which depends largely on water availability and pH conditions, photodegradation is more sensitive to light exposure and atmospheric conditions.
Comparison of Reaction Conditions
Hydrolysis occurs primarily in aqueous environments and requires specific pH levels, usually accelerated in acidic or basic conditions, whereas photodegradation takes place under exposure to UV or visible light, often requiring oxygen or photosensitizers to proceed efficiently. Hydrolysis reactions typically proceed at moderate temperatures and depend on water availability, while photodegradation relies on light intensity and wavelength, often happening more rapidly under direct sunlight. Both processes vary in reaction times, with hydrolysis being slower in many cases, while photodegradation can rapidly break down compounds in surface environments exposed to sunlight.
Environmental Impact and Applications
Hydrolysis and photodegradation both play crucial roles in the breakdown of pollutants but differ significantly in their environmental impact and applications. Hydrolysis, driven by water molecules, is essential for decomposing pesticides and industrial chemicals in aquatic ecosystems, often resulting in less toxic byproducts that reduce long-term environmental harm. Photodegradation relies on sunlight to break down organic contaminants in soil and water, making it vital for natural remediation processes and advanced treatment technologies in wastewater management.
Analytical Methods for Monitoring
Analytical methods for monitoring hydrolysis commonly involve high-performance liquid chromatography (HPLC) and mass spectrometry (MS) to detect and quantify the breakdown products of chemical compounds in aqueous environments. Photodegradation analysis often utilizes UV-Vis spectroscopy and advanced chromatographic techniques coupled with photometric detectors to evaluate changes in chemical structure caused by light exposure. Both methods require precise sample preparation and controlled conditions to accurately assess reaction kinetics and degradation pathways.
Summary: Choosing Between Hydrolysis and Photodegradation
Hydrolysis and photodegradation serve as vital degradation pathways in environmental chemistry, with hydrolysis involving chemical breakdown through water and photodegradation relying on light-induced reactions. Selecting between hydrolysis and photodegradation depends on factors like the chemical nature of the compound, environmental conditions such as moisture presence for hydrolysis, or sunlight exposure for photodegradation. Understanding degradation rates, reaction mechanisms, and application environments guides optimal use for pollutant removal or chemical stability assessments.
Hydrolysis Infographic
 libterm.com
libterm.com