Oxidation is a chemical reaction where a substance loses electrons, often involving the interaction with oxygen. This process plays a crucial role in various natural and industrial contexts, including metabolism, corrosion, and energy production. Discover how understanding oxidation can impact your knowledge of chemistry and everyday life by reading the rest of the article.
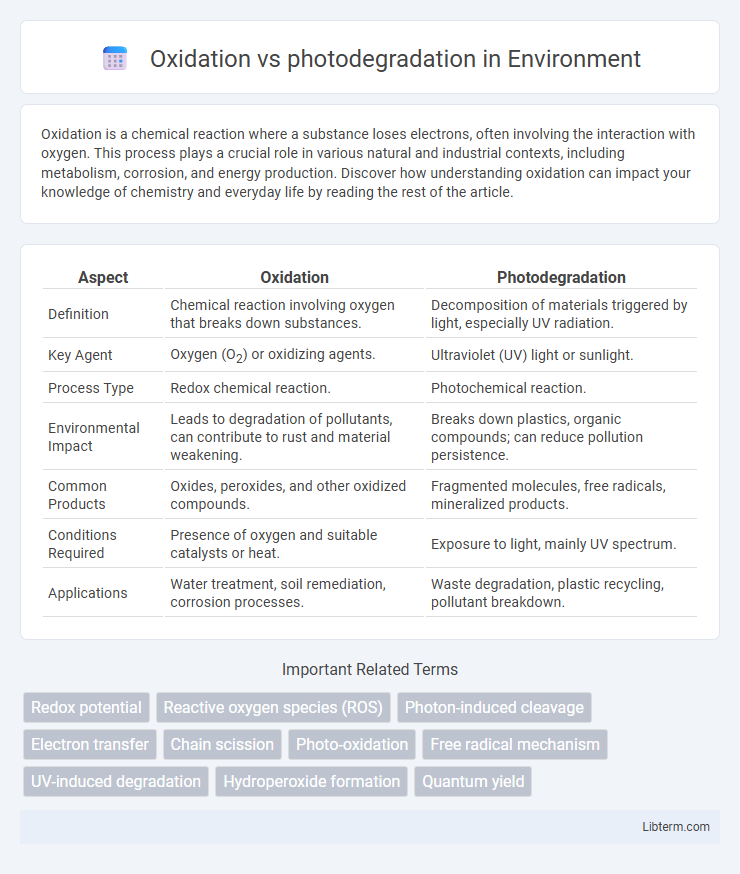
Table of Comparison
| Aspect | Oxidation | Photodegradation |
|---|---|---|
| Definition | Chemical reaction involving oxygen that breaks down substances. | Decomposition of materials triggered by light, especially UV radiation. |
| Key Agent | Oxygen (O2) or oxidizing agents. | Ultraviolet (UV) light or sunlight. |
| Process Type | Redox chemical reaction. | Photochemical reaction. |
| Environmental Impact | Leads to degradation of pollutants, can contribute to rust and material weakening. | Breaks down plastics, organic compounds; can reduce pollution persistence. |
| Common Products | Oxides, peroxides, and other oxidized compounds. | Fragmented molecules, free radicals, mineralized products. |
| Conditions Required | Presence of oxygen and suitable catalysts or heat. | Exposure to light, mainly UV spectrum. |
| Applications | Water treatment, soil remediation, corrosion processes. | Waste degradation, plastic recycling, pollutant breakdown. |
Introduction to Oxidation and Photodegradation
Oxidation is a chemical reaction involving the transfer of electrons, typically resulting in the formation of oxides when substances react with oxygen. Photodegradation refers to the breakdown of materials through exposure to light, especially ultraviolet (UV) radiation, causing chemical changes that alter the material's structure. Both processes are critical in environmental chemistry and material science, influencing the stability and lifespan of organic and inorganic compounds.
Definition and Mechanisms of Oxidation
Oxidation is a chemical reaction where a substance loses electrons, often involving oxygen or other oxidizing agents, leading to the formation of oxides or altered molecular structures. This process typically occurs through mechanisms such as radical formation, electron transfer, or interaction with reactive oxygen species (ROS) like singlet oxygen and hydroxyl radicals. In contrast, photodegradation involves the breakdown of materials via absorption of light energy, triggering molecular changes primarily through photochemical reactions rather than electron loss.
Understanding Photodegradation Processes
Photodegradation involves the breakdown of materials through the absorption of ultraviolet or visible light, leading to molecular changes and the formation of reactive species such as free radicals. Unlike oxidation, which primarily depends on the chemical reaction between oxygen and a substance, photodegradation necessitates photon energy to initiate these transformations. Understanding photodegradation processes is crucial in fields like polymer chemistry and environmental science, where light-induced degradation affects material lifespan and pollutant behavior.
Key Differences Between Oxidation and Photodegradation
Oxidation involves the chemical reaction of a substance with oxygen, leading to changes in molecular structure and often resulting in rust or decay, primarily driven by factors such as heat, moisture, and catalysts. Photodegradation refers to the breakdown of materials caused by exposure to ultraviolet (UV) light, where photon energy induces molecular bond cleavage and generates reactive species like free radicals. Key differences include oxidation's dependence on oxygen and chemical reactions, whereas photodegradation depends on light energy and photochemical processes, impacting material stability, lifetime, and degradation pathways distinctly.
Chemical Reactions Involved in Each Process
Oxidation involves chemical reactions where oxygen or reactive oxygen species (ROS) interact with a substance, resulting in electron loss and the formation of oxides or peroxides, commonly seen in metal corrosion and organic compound degradation. Photodegradation, on the other hand, occurs when photons from ultraviolet or visible light induce bond cleavage or structural changes in molecules, often generating free radicals that accelerate breakdown. Both processes rely on radical intermediates, but oxidation primarily depends on oxygen species, whereas photodegradation is driven by light energy triggering direct molecular excitation.
Environmental Factors Influencing Oxidation and Photodegradation
Environmental factors such as temperature, humidity, and exposure to ultraviolet (UV) radiation significantly influence oxidation and photodegradation processes. Oxidation is accelerated by higher temperatures and the presence of oxygen, while photodegradation is predominantly driven by UV light intensity and wavelength. Material composition and atmospheric conditions, including pollutant levels and moisture, also play critical roles in determining the rate and extent of both degradation mechanisms.
Real-World Examples and Applications
Oxidation plays a crucial role in the degradation of metals, such as the rusting of iron in outdoor structures, which compromises structural integrity over time. Photodegradation is prominent in polymer materials exposed to UV radiation, like the fading of plastics and paint in automotive exteriors, leading to surface cracking and discoloration. Real-world applications harness oxidation in wastewater treatment to break down pollutants, while photodegradation is utilized in environmental cleanup through sunlight-driven degradation of pesticides.
Impact on Material Stability and Longevity
Oxidation causes chemical breakdown by reacting with oxygen, leading to changes in material properties such as brittleness and discoloration, which reduce stability and longevity. Photodegradation results from the absorption of ultraviolet (UV) light, triggering molecular bond cleavage that weakens materials and accelerates surface degradation. The combined effects of oxidation and photodegradation significantly shorten the lifespan of polymers, coatings, and organic materials by compromising structural integrity and appearance.
Prevention and Mitigation Strategies
Prevention and mitigation strategies for oxidation include using antioxidants, controlling temperature, and minimizing oxygen exposure to protect materials from oxidative damage. Photodegradation prevention involves applying UV stabilizers, protective coatings, and using packaging that blocks or filters harmful light wavelengths. Combining these approaches ensures enhanced durability and longevity of products exposed to environmental stressors such as air, heat, and sunlight.
Future Research Trends and Innovations
Future research trends in oxidation and photodegradation focus on developing advanced catalysts and novel nanomaterials to enhance reaction efficiency and selectivity. Innovations emphasize integrating photocatalytic processes with renewable energy sources to achieve sustainable environmental remediation. Emerging studies explore hybrid oxidation-photodegradation systems leveraging artificial intelligence for real-time monitoring and process optimization.
Oxidation Infographic
 libterm.com
libterm.com