Thermal degradation occurs when materials break down due to excessive heat, leading to chemical changes that can weaken their structure and performance. Understanding how heat affects polymers, plastics, and other substances is crucial for improving product durability and safety. Explore the rest of the article to learn how thermal degradation impacts your materials and ways to prevent it.
Table of Comparison
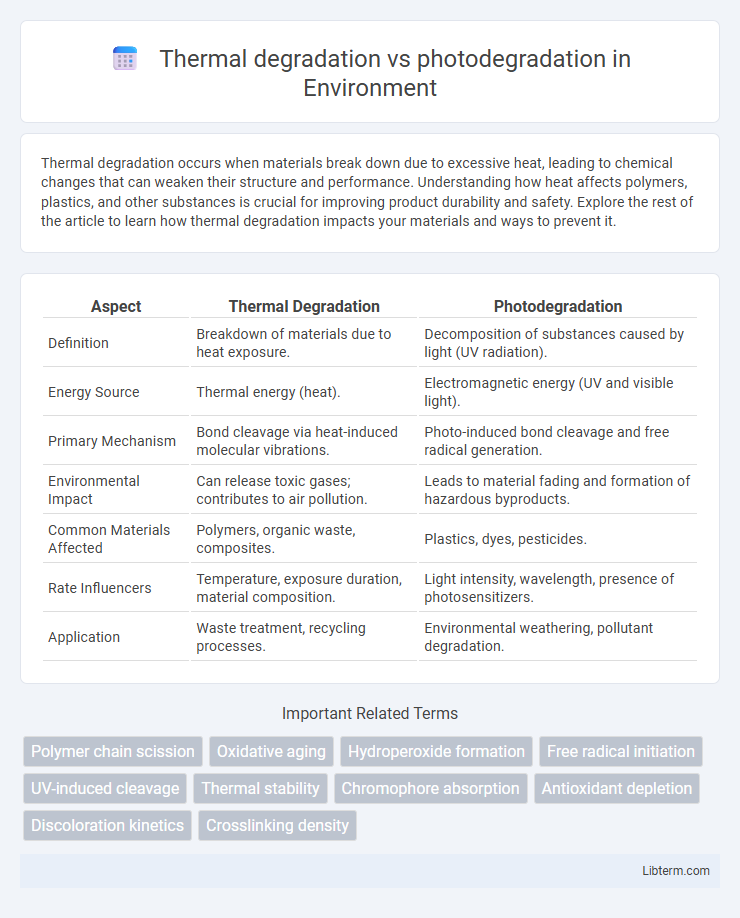
| Aspect | Thermal Degradation | Photodegradation |
|---|---|---|
| Definition | Breakdown of materials due to heat exposure. | Decomposition of substances caused by light (UV radiation). |
| Energy Source | Thermal energy (heat). | Electromagnetic energy (UV and visible light). |
| Primary Mechanism | Bond cleavage via heat-induced molecular vibrations. | Photo-induced bond cleavage and free radical generation. |
| Environmental Impact | Can release toxic gases; contributes to air pollution. | Leads to material fading and formation of hazardous byproducts. |
| Common Materials Affected | Polymers, organic waste, composites. | Plastics, dyes, pesticides. |
| Rate Influencers | Temperature, exposure duration, material composition. | Light intensity, wavelength, presence of photosensitizers. |
| Application | Waste treatment, recycling processes. | Environmental weathering, pollutant degradation. |
Introduction to Polymer Degradation
Polymer degradation involves chemical or physical changes that deteriorate polymer properties over time, with thermal degradation and photodegradation as primary mechanisms. Thermal degradation occurs when polymers break down due to elevated temperatures, leading to chain scission, cross-linking, or oxidation, significantly affecting materials like polyethylene and polyvinyl chloride. Photodegradation results from ultraviolet (UV) radiation exposure, causing bond cleavage and free radical formation in polymers such as polypropylene and polystyrene, ultimately compromising mechanical strength and stability.
Defining Thermal Degradation
Thermal degradation is the breakdown of materials caused by exposure to elevated temperatures, leading to chemical changes such as bond cleavage and molecular weight reduction. This process results in the loss of mechanical properties and structural integrity in polymers and other organic compounds. Photodegradation, by contrast, involves the decomposition triggered by ultraviolet or visible light, causing photo-oxidative reactions distinct from thermally induced chemical changes.
Understanding Photodegradation
Photodegradation is the breakdown of materials caused by exposure to light, especially ultraviolet (UV) radiation, which leads to chemical changes in polymers, dyes, and other substances. This process involves the absorption of photons that generate free radicals, resulting in molecular bond cleavage and material deterioration. Unlike thermal degradation, which is driven by heat and affects a wide temperature range, photodegradation specifically depends on light intensity, wavelength, and exposure duration, making it critical in assessing the longevity and stability of outdoor materials.
Key Differences Between Thermal and Photodegradation
Thermal degradation involves the breakdown of materials due to elevated temperatures, causing chemical bonds to weaken or break through heat exposure, whereas photodegradation results from exposure to ultraviolet (UV) light, causing molecular changes primarily through photochemical reactions. Thermal degradation typically occurs in polymers at temperatures above their glass transition or melting points, while photodegradation mainly affects surface layers exposed to sunlight or UV radiation. Key differences include the energy source driving the degradation--heat for thermal and UV photons for photodegradation--and the resulting chemical pathways, with thermal degradation favoring pyrolysis and photodegradation leading to free radical formation and chain scission.
Mechanisms of Thermal Degradation
Thermal degradation involves the breakdown of materials through heat-induced chemical reactions such as chain scission, cross-linking, and depolymerization, resulting in the formation of volatile compounds and char. The mechanism typically initiates with bond cleavage caused by elevated temperatures that disrupt the polymer's molecular structure without exposure to light. This process contrasts with photodegradation, where ultraviolet or visible light triggers radical formation and subsequent molecular breakdown.
Mechanisms of Photodegradation
Photodegradation occurs when materials absorb ultraviolet (UV) light, leading to the excitation of electrons and the formation of reactive species such as free radicals and singlet oxygen. These reactive intermediates attack polymer chains, causing bond scission, cross-linking, and changes in molecular structure. Unlike thermal degradation, which primarily involves heat-induced bond cleavage, photodegradation is driven by photon energy that alters chemical bonds specifically through photooxidative reactions.
Factors Influencing Thermal Degradation
Thermal degradation of polymers is primarily influenced by temperature, exposure time, and the chemical structure of the material, with higher temperatures accelerating bond cleavage and volatilization processes. The presence of oxygen and impurities can catalyze oxidation reactions, further destabilizing the polymer matrix during thermal exposure. Polymer morphology, such as crystallinity and molecular weight distribution, also affects the rate and extent of thermal degradation.
Factors Affecting Photodegradation
Photodegradation primarily depends on factors such as light intensity, wavelength, and exposure duration, with ultraviolet (UV) radiation playing a critical role in breaking chemical bonds. Environmental conditions including temperature, oxygen concentration, and the presence of photosensitizers or catalysts can significantly influence the rate and extent of photodegradation. Comparatively, thermal degradation is driven by elevated temperatures causing molecular fragmentation, but it is less dependent on light-related variables than photodegradation.
Applications Impacted by Degradation Processes
Thermal degradation primarily affects polymer applications in automotive and aerospace industries, where high temperatures cause material embrittlement and reduced mechanical performance. Photodegradation significantly impacts outdoor coatings and packaging materials, leading to color fading and loss of barrier properties under prolonged UV exposure. Understanding these degradation mechanisms is crucial for developing durable materials in electronics, construction, and renewable energy sectors.
Prevention and Mitigation Strategies
Thermal degradation prevention involves controlling temperature exposure through insulation, heat sinks, and temperature monitoring systems to reduce material breakdown and prolong lifespan. Photodegradation mitigation focuses on UV stabilizers, protective coatings, and incorporating UV-absorbing additives to shield polymers and organic compounds from light-induced damage. Combining these strategies enhances durability by limiting oxidative stress and photochemical reactions in materials exposed to heat and sunlight.
Thermal degradation Infographic
 libterm.com
libterm.com