ug/L (micrograms per liter) is a unit of measurement used to express the concentration of a substance in a liquid, commonly in water quality testing and environmental monitoring. Understanding this metric is essential for interpreting contaminant levels and ensuring they meet safety standards. Explore the article to learn more about how ug/L measurements impact your health and environment.
Table of Comparison
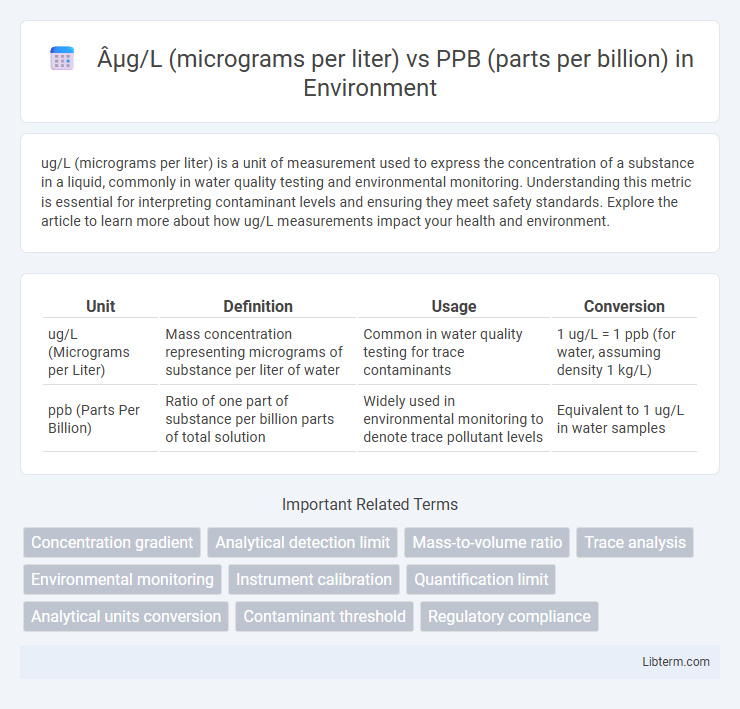
| Unit | Definition | Usage | Conversion |
|---|---|---|---|
| ug/L (Micrograms per Liter) | Mass concentration representing micrograms of substance per liter of water | Common in water quality testing for trace contaminants | 1 ug/L = 1 ppb (for water, assuming density 1 kg/L) |
| ppb (Parts Per Billion) | Ratio of one part of substance per billion parts of total solution | Widely used in environmental monitoring to denote trace pollutant levels | Equivalent to 1 ug/L in water samples |
Introduction to µg/L and PPB
Micrograms per liter (ug/L) and parts per billion (PPB) are both units used to measure extremely low concentrations of substances in liquids, often in environmental science and water quality testing. One ug/L is equivalent to one PPB when measuring dilute aqueous solutions, as they both represent one unit of solute per billion units of solution by mass or volume. Understanding the interchangeability of ug/L and PPB helps ensure accurate communication and interpretation of trace contaminant data in environmental monitoring and regulatory standards.
Definition of µg/L (Micrograms per Liter)
ug/L (micrograms per liter) is a unit of concentration representing one microgram of a substance dissolved in one liter of liquid, commonly used in water quality analysis and environmental testing. This unit directly measures mass per volume, facilitating precise quantification of trace contaminants such as heavy metals and pollutants in aqueous solutions. Although ug/L and ppb (parts per billion) are numerically equivalent for water measurements due to the density of water being close to 1 kg/L, ug/L provides a clear mass-based concentration essential for regulatory compliance and scientific reporting.
Understanding Parts per Billion (PPB)
Parts per billion (PPB) is a unit of measurement used to express the concentration of a substance in water or air, equivalent to micrograms per liter (ug/L) when measuring dilute aqueous solutions. One PPB signifies one part of a substance per billion parts of the total solution, making it a crucial metric in environmental science for detecting trace contaminants. Understanding PPB helps accurately assess pollutant levels and ensures compliance with health and safety standards in water quality monitoring.
Conversion Between µg/L and PPB
Conversion between ug/L (micrograms per liter) and PPB (parts per billion) is straightforward in aqueous solutions because 1 ug/L is equivalent to 1 PPB. This equivalence assumes the density of the solution is close to that of water (1 g/mL), making the units directly interchangeable when measuring contaminants in water. For substances dissolved in water, monitoring pollutant levels can use either ug/L or PPB without altering the numeric value, facilitating consistent reporting in environmental science and water quality analysis.
Relevance in Environmental Science
Micrograms per liter (ug/L) and parts per billion (PPB) are equivalent units used to express trace concentrations of contaminants in water and air, crucial for environmental monitoring. Both units provide precise measurements for pollutants like heavy metals, pesticides, and other toxic substances, aiding regulatory compliance and risk assessment. Accurate conversion between ug/L and PPB ensures consistent data interpretation in environmental science and pollution control strategies.
Applications in Water Quality Testing
In water quality testing, ug/L and parts per billion (PPB) are used interchangeably to measure trace contaminants like heavy metals and pesticides. Both units quantify the concentration of substances at very low levels, essential for assessing compliance with environmental standards set by agencies such as the EPA. Accurate detection of ug/L or PPB ensures safe drinking water by monitoring pollutants that can cause health hazards even at trace concentrations.
Usage in Pharmaceutical Analysis
In pharmaceutical analysis, ug/L and PPB are often used interchangeably since 1 ug/L equals 1 PPB, both representing trace-level concentrations of substances in liquid samples. Accurate measurement of active pharmaceutical ingredients and impurities at these low levels is critical for quality control and regulatory compliance. Instruments such as ICP-MS and LC-MS/MS commonly report results in ug/L or PPB to ensure precise quantification of contaminants and residues.
Accuracy and Measurement Challenges
ug/L (micrograms per liter) and PPB (parts per billion) are often used interchangeably in water quality analysis, as 1 ug/L typically equals 1 PPB for aqueous solutions, assuming water's density close to 1 g/mL. Accuracy in these measurements depends on instrumentation sensitivity, calibration standards, and matrix effects, where trace contaminant detection at such low concentrations can be affected by sample contamination or instrument drift. Measurement challenges include maintaining detection limits consistent with regulatory requirements and addressing interferences that may skew results, necessitating advanced analytical techniques like ICP-MS or high-resolution chromatography.
Regulatory Standards and Guidelines
Regulatory standards often use ug/L and PPB interchangeably to measure contaminant concentrations in water, with both units representing the same quantity of one microgram per liter of water, equivalent to one part per billion for dilute aqueous solutions. Agencies such as the U.S. Environmental Protection Agency (EPA) and the World Health Organization (WHO) set maximum contaminant levels (MCLs) using ug/L or PPB, ensuring consistent monitoring and compliance across various pollutants like heavy metals and pesticides. Accurate understanding of these units is crucial for interpreting water quality data and adhering to regulatory guidelines designed to protect public health.
Choosing the Right Unit: µg/L vs PPB
Choosing the right unit between ug/L (micrograms per liter) and PPB (parts per billion) depends on the context of measurement, as both represent equivalent concentrations where 1 ug/L equals 1 PPB in water. For precise water quality analysis, ug/L offers clarity and aligns with standardized reporting in environmental regulations. Consider using ug/L for scientific documentation and PPB for communication with broader audiences, ensuring consistent understanding across disciplines.
µg/L (micrograms per liter) Infographic
 libterm.com
libterm.com