Mg/L (milligrams per liter) is a common unit of measurement used to express the concentration of a substance in a liquid, often applied in water quality testing and chemical analysis. Understanding mg/L is crucial for interpreting data on dissolved solids, pollutants, or nutrients in water, ensuring safety and compliance with environmental standards. Explore the rest of this article to learn how mg/L impacts your water quality assessments and health.
Table of Comparison
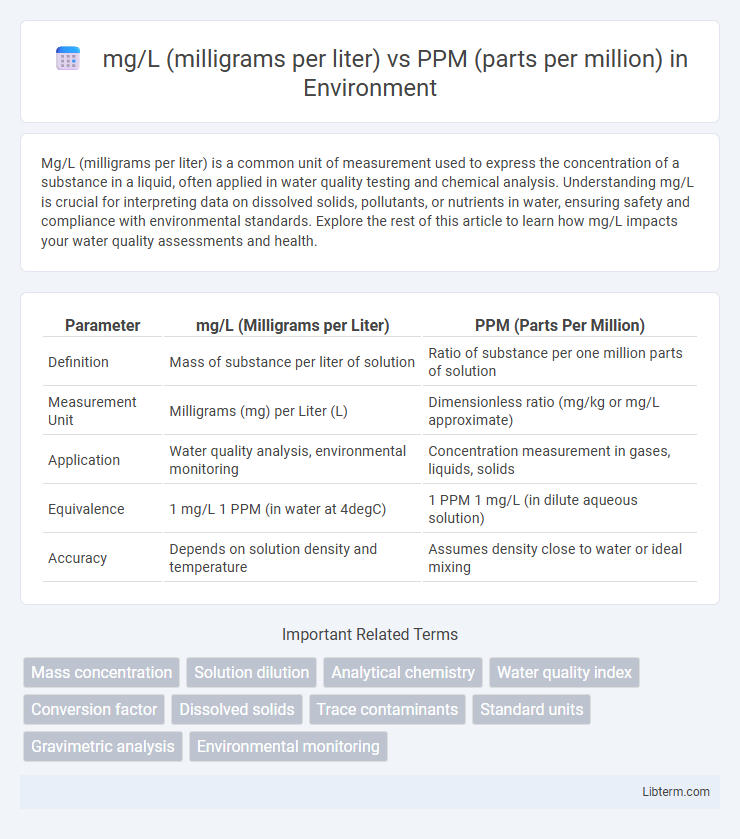
| Parameter | mg/L (Milligrams per Liter) | PPM (Parts Per Million) |
|---|---|---|
| Definition | Mass of substance per liter of solution | Ratio of substance per one million parts of solution |
| Measurement Unit | Milligrams (mg) per Liter (L) | Dimensionless ratio (mg/kg or mg/L approximate) |
| Application | Water quality analysis, environmental monitoring | Concentration measurement in gases, liquids, solids |
| Equivalence | 1 mg/L 1 PPM (in water at 4degC) | 1 PPM 1 mg/L (in dilute aqueous solution) |
| Accuracy | Depends on solution density and temperature | Assumes density close to water or ideal mixing |
Understanding mg/L and PPM: Basic Definitions
Milligrams per liter (mg/L) and parts per million (PPM) both measure substance concentration in water, with 1 mg/L equivalent to 1 PPM in dilute aqueous solutions due to water's density. Mg/L represents the mass of a solute in milligrams dissolved in one liter of solution, providing a direct measure of concentration by weight. PPM expresses the ratio of one part of substance to one million parts of total solution, often used interchangeably with mg/L in environmental and chemical analysis contexts.
The Science Behind Concentration Units
Milligrams per liter (mg/L) and parts per million (ppm) are both units used to express concentrations in solutions, especially in water quality analysis. Scientifically, 1 mg/L equals 1 ppm when measuring dilute aqueous solutions, as both represent one part of solute per one million parts of solution by mass or volume. Understanding this equivalence is crucial for accurately interpreting concentration data in environmental science, chemistry, and engineering applications.
How to Convert mg/L to PPM
Converting mg/L (milligrams per liter) to PPM (parts per million) involves understanding that both units express concentration in water-based solutions equivalently. Since 1 mg/L is equal to 1 PPM when the density of the solution is comparable to water (1 kg/L), the conversion is straightforward: 1 mg/L = 1 PPM. This equivalence applies primarily to dilute aqueous solutions where the solvent density approximates that of water.
Real-World Applications: Where mg/L and PPM Matter
Milligrams per liter (mg/L) and parts per million (PPM) are critical units in water quality testing, environmental monitoring, and chemical analysis, where precise measurement of contaminants like heavy metals, nitrates, and organic compounds directly impacts human health and ecosystem safety. In drinking water regulations, mg/L and PPM help ensure pollutant concentrations remain below harmful thresholds, such as the EPA's maximum contaminant level for lead (0.015 mg/L or 15 PPM). Industrial processes and wastewater treatment plants rely on these units for real-time monitoring to maintain compliance with environmental standards and optimize treatment performance.
Key Differences Between mg/L and PPM
Mg/L (milligrams per liter) and PPM (parts per million) both measure concentration levels in liquids, where 1 mg/L equals 1 PPM in water-based solutions due to the density of water being close to 1 g/mL. Mg/L specifically quantifies mass per volume, making it more precise for substances dissolved in water, while PPM expresses a ratio that can apply to various contexts like air, soil, and water. Understanding this distinction is essential for accurate data interpretation in environmental monitoring, water quality analysis, and chemical measurements.
Usage in Water Quality Analysis
Milligrams per liter (mg/L) and parts per million (PPM) are commonly used units in water quality analysis to measure the concentration of contaminants or substances. Both units are effectively equivalent for dilute aqueous solutions, where 1 mg/L equals 1 PPM, enabling precise monitoring of pollutants such as heavy metals, nutrients, and chemical additives. Accurate use of mg/L and PPM helps in assessing compliance with environmental standards and ensuring safe drinking water quality.
Significance in Environmental Monitoring
Milligrams per liter (mg/L) and parts per million (ppm) are commonly used units in environmental monitoring to measure the concentration of pollutants in water, reflecting nearly equivalent values for dilute aqueous solutions. These units are significant for assessing water quality parameters such as dissolved oxygen, nutrients, and contaminants, enabling regulatory compliance and ecosystem health evaluation. Accurate measurement in mg/L or ppm supports effective decision-making in pollution control and environmental risk assessment.
Laboratory Practices: Measurement and Reporting
In laboratory practices, mg/L and PPM are often used interchangeably to express the concentration of substances in aqueous solutions, where 1 mg/L equals 1 PPM due to the density of water being approximately 1 g/mL. Accurate calibration of analytical instruments such as spectrophotometers and ion-selective electrodes ensures precise measurement and reliable reporting of these units. Standard operating procedures typically emphasize unit consistency and traceability to certified reference materials for maintaining data integrity in environmental and industrial testing.
Common Misconceptions About mg/L and PPM
Milligrams per liter (mg/L) and parts per million (PPM) are often mistakenly treated as interchangeable units, but mg/L measures mass concentration while PPM is a dimensionless ratio representing parts of a substance per million parts of the total. In aqueous solutions, 1 mg/L usually equals 1 PPM due to the density of water being close to 1 kg/L, yet this equivalence fails in non-aqueous or mixed density systems. Confusion arises when concentrations are reported without specifying the medium or assuming the conversion holds for all substances and conditions.
Choosing the Right Unit for Your Needs
Milligrams per liter (mg/L) and parts per million (PPM) both express concentration levels in water and solutions with nearly equivalent values, where 1 mg/L typically equals 1 PPM for dilute aqueous solutions. Selecting mg/L is preferable for laboratory analysis and regulatory compliance as it directly quantifies mass per volume, simplifying calculations in chemical dosing and water quality testing. PPM is often favored in environmental monitoring and everyday communication due to its intuitive scale for trace contaminants, but understanding the slight contextual differences ensures accurate data interpretation for specific applications.
mg/L (milligrams per liter) Infographic
 libterm.com
libterm.com