Marl is a sedimentary rock composed primarily of clay and calcium carbonate, often used to improve soil quality in agriculture by enhancing nutrient retention and drainage. This natural mineral is essential for gardeners and farmers looking to balance pH levels and boost plant growth. Discover how marl can benefit your land and gardening projects by reading the full article.
Table of Comparison
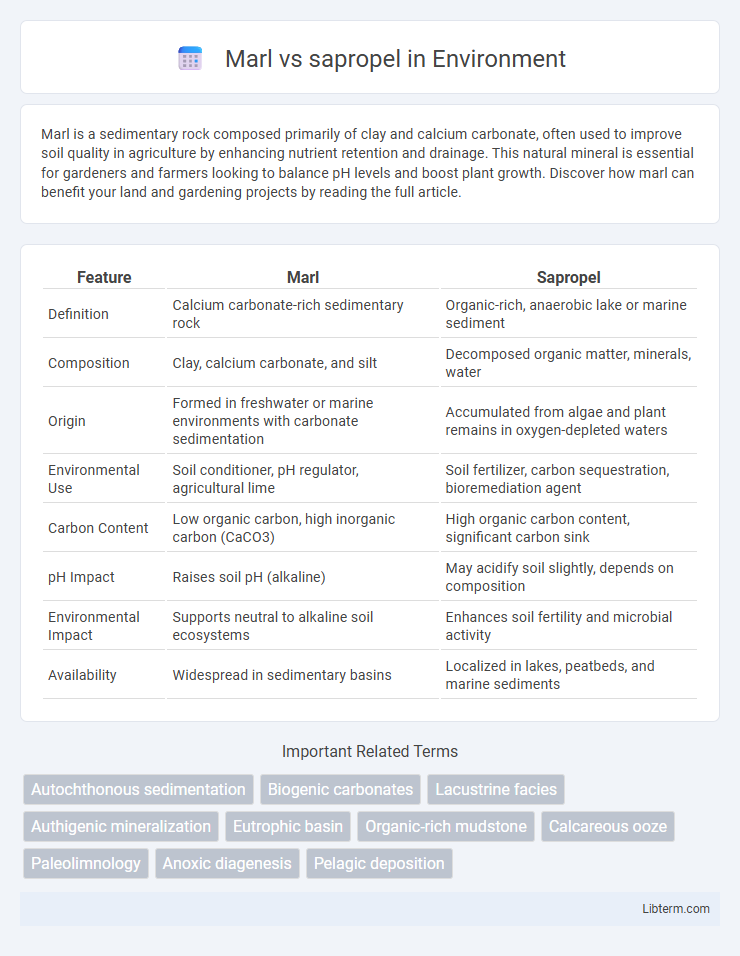
| Feature | Marl | Sapropel |
|---|---|---|
| Definition | Calcium carbonate-rich sedimentary rock | Organic-rich, anaerobic lake or marine sediment |
| Composition | Clay, calcium carbonate, and silt | Decomposed organic matter, minerals, water |
| Origin | Formed in freshwater or marine environments with carbonate sedimentation | Accumulated from algae and plant remains in oxygen-depleted waters |
| Environmental Use | Soil conditioner, pH regulator, agricultural lime | Soil fertilizer, carbon sequestration, bioremediation agent |
| Carbon Content | Low organic carbon, high inorganic carbon (CaCO3) | High organic carbon content, significant carbon sink |
| pH Impact | Raises soil pH (alkaline) | May acidify soil slightly, depends on composition |
| Environmental Impact | Supports neutral to alkaline soil ecosystems | Enhances soil fertility and microbial activity |
| Availability | Widespread in sedimentary basins | Localized in lakes, peatbeds, and marine sediments |
Introduction to Marl and Sapropel
Marl is a sedimentary rock composed primarily of calcium carbonate mixed with clay and silt, often used in agriculture to improve soil quality and pH balance. Sapropel consists of organic-rich, fine-grained sediment formed in aquatic environments through the accumulation of decomposed plant and animal material, providing essential nutrients for enhancing soil fertility. Both marl and sapropel serve as natural soil amendments but differ significantly in their composition and origin, influencing their applications in environmental and agricultural contexts.
Formation Processes of Marl and Sapropel
Marl forms through the chemical precipitation of calcium carbonate and clay minerals in freshwater or marine environments, often influenced by biological activity and sedimentation rates. Sapropel develops from the accumulation of organic-rich sediments in anoxic or low-oxygen water conditions, where the decay of organic matter is slowed, leading to high carbon content. Differences in redox conditions and sediment composition distinctly govern the formation processes of both marl and sapropel.
Geological Distribution and Occurrence
Marl predominantly occurs in lacustrine and marine environments, with significant deposits found across Europe, North America, and parts of Asia, often associated with limestone formations from the Paleogene and Neogene periods. Sapropel is typically found in anoxic conditions of marine basins and lakes, especially in the Mediterranean Sea, Baltic Sea, and Black Sea regions, formed during interglacial periods rich in organic matter. Both marl and sapropel layers serve as important geological indicators of past environmental conditions and sedimentation processes.
Chemical and Mineral Composition
Marl primarily consists of calcium carbonate (CaCO3), clay minerals, and varying amounts of silica, with its composition often influenced by the presence of calcium-rich sediments. Sapropel, on the other hand, is rich in organic matter, including decomposed plant and animal material, and contains minerals such as clay, silt, and a significant concentration of humic substances. The chemical profile of marl is dominated by its carbonate content, while sapropel's composition reflects high levels of phosphorus, nitrogen, and carbon due to organic accumulation.
Physical Characteristics and Properties
Marl is a sedimentary material composed primarily of calcium carbonate mixed with clay and silt, exhibiting a loose, crumbly texture and light gray to greenish color, whereas sapropel consists of organic-rich, fine-grained sediments characterized by a dark brown to black hue with high moisture content. Marl has moderate permeability and a relatively alkaline pH due to its carbonate content, while sapropel displays low permeability, higher water retention, and strong reducing conditions caused by decomposed organic matter. The physical differences influence their applications in agriculture and environmental management, with marl often used as a soil amendment to neutralize acidity, and sapropel valued for its nutrient-rich composition in soil fertilization.
Environmental Roles and Significance
Marl plays a crucial role in buffering acidic waters and supporting aquatic ecosystems by providing essential calcium carbonate, which helps maintain pH balance and promotes biodiversity. Sapropel, rich in organic matter, is vital for nutrient cycling in aquatic environments, enhancing sediment fertility and supporting microbial communities that contribute to carbon sequestration. Both marl and sapropel significantly influence sediment chemistry and habitat quality, shaping the ecological dynamics of freshwater and marine systems.
Agricultural and Industrial Applications
Marl, a calcium carbonate-rich sediment, improves soil pH and fertility in agricultural applications, enhancing crop yields by neutralizing acidic soils. Sapropel, an organic-rich sediment formed in freshwater lakes, acts as a natural fertilizer due to its high nutrient content, boosting soil microbial activity and plant growth. Industrially, marl is used in cement and brick production, while sapropel finds applications in cosmetics and biostimulants, leveraging its organic compounds for sustainable product formulations.
Extraction and Processing Methods
Marl extraction typically involves open-pit mining where the soft, calcareous sediment is dredged or excavated from lakebeds or quarries, followed by drying and grinding to produce a fine powder. Sapropel is extracted through sediment sampling or dredging from water bodies, then dewatered and dried to remove moisture content before further processing. Both materials undergo size reduction and purification processes tailored for agricultural or environmental applications, optimizing nutrient availability and soil amendment efficiency.
Comparative Analysis: Marl vs Sapropel
Marl consists primarily of calcium carbonate mixed with clay and silt, making it ideal for soil conditioning and pH adjustment, while sapropel is an organic-rich sediment formed from decomposed aquatic plants, rich in nutrients like nitrogen and phosphorus. Marl improves soil structure and provides essential minerals for plant growth, whereas sapropel enhances soil fertility by increasing organic matter content and moisture retention. The choice between marl and sapropel depends on specific agricultural or environmental needs, with marl favored for liming acidic soils and sapropel used as a natural fertilizer and soil conditioner.
Future Prospects and Research Directions
Future research on marl and sapropel focuses on enhancing their applications in agriculture and environmental sustainability. Advances in biogeochemical analysis and sedimentology could unlock new insights into nutrient cycling and carbon sequestration potentials of these materials. Innovative methods aim to optimize marl and sapropel for soil amendment and climate change mitigation strategies.
Marl Infographic
 libterm.com
libterm.com