Methane is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide, making its emission reduction critical in combating climate change. It is produced through natural processes like wetlands and human activities including agriculture, fossil fuel extraction, and waste management. Explore the rest of the article to understand how controlling methane emissions can protect your environment and the planet's future.
Table of Comparison
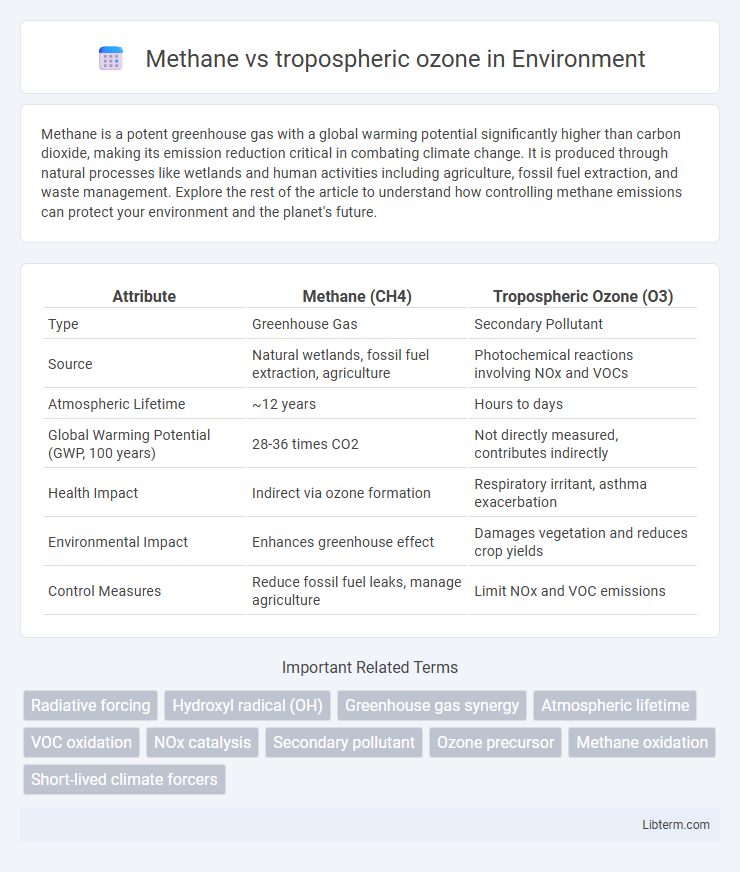
| Attribute | Methane (CH4) | Tropospheric Ozone (O3) |
|---|---|---|
| Type | Greenhouse Gas | Secondary Pollutant |
| Source | Natural wetlands, fossil fuel extraction, agriculture | Photochemical reactions involving NOx and VOCs |
| Atmospheric Lifetime | ~12 years | Hours to days |
| Global Warming Potential (GWP, 100 years) | 28-36 times CO2 | Not directly measured, contributes indirectly |
| Health Impact | Indirect via ozone formation | Respiratory irritant, asthma exacerbation |
| Environmental Impact | Enhances greenhouse effect | Damages vegetation and reduces crop yields |
| Control Measures | Reduce fossil fuel leaks, manage agriculture | Limit NOx and VOC emissions |
Introduction to Methane and Tropospheric Ozone
Methane (CH4) is a potent greenhouse gas with a global warming potential approximately 28-36 times greater than carbon dioxide over a 100-year period, significantly contributing to climate change. Tropospheric ozone (O3), formed by photochemical reactions involving methane and other precursor pollutants such as nitrogen oxides (NOx) and volatile organic compounds (VOCs), acts as a significant short-lived climate pollutant and air quality hazard. Understanding the interactions and sources of methane and tropospheric ozone is crucial for developing effective climate mitigation and air pollution control strategies.
Chemical Properties and Structure
Methane (CH4) is a stable tetrahedral molecule with a single carbon atom bonded to four hydrogen atoms via strong sigma bonds, making it relatively inert under tropospheric conditions. Tropospheric ozone (O3) is a triatomic molecule with a bent angular structure, exhibiting high reactivity due to its resonance-stabilized diradical nature and ability to act as a strong oxidant. The distinct molecular geometries and bond energies of methane and tropospheric ozone define their contrasting chemical behaviors and roles in atmospheric chemistry.
Sources and Emission Pathways
Methane primarily originates from natural wetlands, livestock digestion, fossil fuel extraction, and waste management, contributing significantly to greenhouse gas emissions. Tropospheric ozone is not emitted directly but forms through photochemical reactions involving precursor gases such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) emitted from vehicles, industrial activities, and biomass burning. Emission pathways for methane release involve anaerobic decomposition and fossil fuel leaks, while tropospheric ozone formation depends on sunlight-driven reactions in the lower atmosphere.
Atmospheric Lifetimes and Distribution
Methane has an atmospheric lifetime of approximately 12 years, allowing it to mix globally and influence both the troposphere and stratosphere, while tropospheric ozone exhibits a much shorter lifetime, ranging from hours to weeks, leading to highly variable and localized distributions. Methane's longevity contributes significantly to its role as a long-lived greenhouse gas, whereas tropospheric ozone is a secondary pollutant formed by photochemical reactions involving precursors like NOx and VOCs. The spatial distribution of tropospheric ozone is closely linked to emission sources and sunlight, exhibiting higher concentrations in polluted urban and industrial regions compared to the more uniform global presence of methane.
Roles as Greenhouse Gases
Methane (CH4) is a potent greenhouse gas with a global warming potential approximately 28-36 times greater than carbon dioxide over a 100-year period, primarily influencing climate change by trapping heat in the atmosphere. Tropospheric ozone (O3) acts as a secondary greenhouse gas formed through photochemical reactions involving precursors like nitrogen oxides (NOx) and volatile organic compounds (VOCs), contributing to radiative forcing and warming near the Earth's surface. Both gases play critical roles in atmospheric chemistry and climate systems, with methane being a long-lived forcing agent and tropospheric ozone representing a short-lived climate pollutant with significant regional variability.
Impacts on Air Quality and Human Health
Methane is a significant precursor to tropospheric ozone, which is a major air pollutant linked to respiratory problems and cardiovascular diseases. Elevated levels of tropospheric ozone degrade air quality by contributing to smog formation and exacerbating asthma and other lung conditions. Reducing methane emissions effectively lowers tropospheric ozone concentrations, thereby improving public health outcomes and decreasing hospital admissions related to air pollution.
Interactions and Feedback Mechanisms
Methane significantly influences tropospheric ozone formation through photochemical reactions involving hydroxyl radicals, increasing ozone concentrations that contribute to warming and air pollution. Elevated tropospheric ozone levels can alter atmospheric oxidizing capacity, affecting methane's atmospheric lifetime and creating a feedback loop that amplifies greenhouse gas effects. These interactions underscore the importance of controlling methane emissions to mitigate tropospheric ozone pollution and climate change impacts.
Contribution to Climate Forcing
Methane is a potent greenhouse gas with a global warming potential approximately 28-36 times greater than CO2 over a 100-year period, making it a significant contributor to climate forcing. Tropospheric ozone, formed by the reaction of sunlight with pollutants such as methane and nitrogen oxides, acts as a secondary greenhouse gas, contributing to radiative forcing estimated between 0.4 to 0.8 W/m2. While methane directly adds to climate warming, its role in producing tropospheric ozone amplifies its impact on atmospheric chemistry and climate forcing.
Mitigation Strategies and Policy Approaches
Methane mitigation strategies prioritize reducing emissions from agriculture, landfills, and fossil fuel extraction, utilizing technologies such as improved leak detection and capture systems. Tropospheric ozone is controlled by limiting precursor emissions, including nitrogen oxides (NOx) and volatile organic compounds (VOCs), through stricter vehicle emission standards and industrial regulations. Policy approaches integrate multi-pollutant frameworks targeting both methane and ozone precursors, supported by international agreements like the Global Methane Pledge and regional air quality management plans.
Future Outlook and Research Directions
Methane continues to be a critical target for climate mitigation due to its high global warming potential and impact on tropospheric ozone formation, which exacerbates air quality and health issues. Future research explores advanced methane detection technologies, emission reduction strategies, and improved atmospheric chemical models to better predict methane-ozone interactions. Enhanced interdisciplinary studies will focus on linking methane mitigation with tropospheric ozone management to optimize climate and public health policies.
Methane Infographic
 libterm.com
libterm.com