Alkaline soil has a pH level above 7, which can limit the availability of essential nutrients like iron, manganese, and phosphorus, affecting plant growth. Certain plants thrive in these conditions, but most prefer neutral or slightly acidic soil for optimal nutrient absorption. Discover how to manage alkaline soil effectively to improve Your garden's health by reading the rest of the article.
Table of Comparison
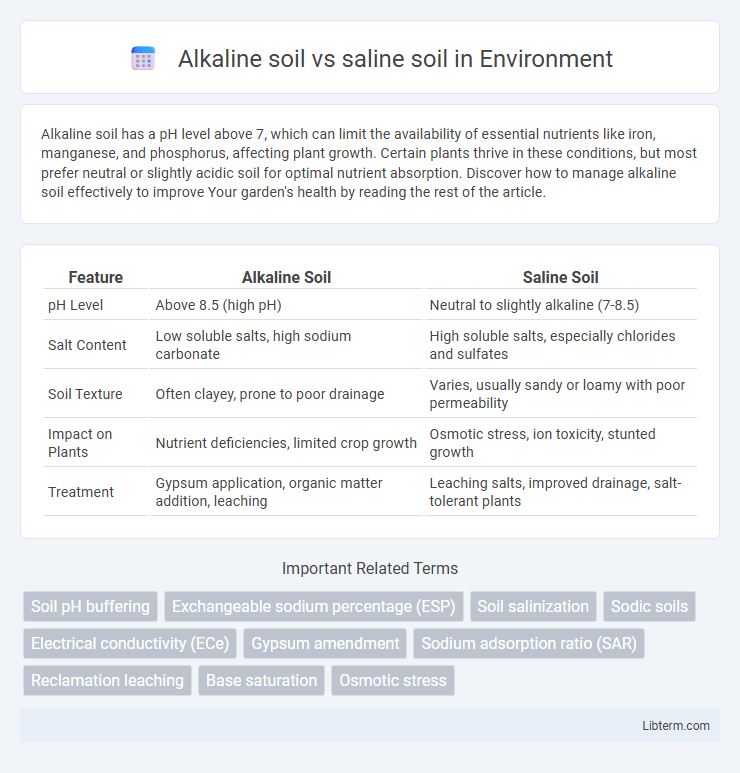
| Feature | Alkaline Soil | Saline Soil |
|---|---|---|
| pH Level | Above 8.5 (high pH) | Neutral to slightly alkaline (7-8.5) |
| Salt Content | Low soluble salts, high sodium carbonate | High soluble salts, especially chlorides and sulfates |
| Soil Texture | Often clayey, prone to poor drainage | Varies, usually sandy or loamy with poor permeability |
| Impact on Plants | Nutrient deficiencies, limited crop growth | Osmotic stress, ion toxicity, stunted growth |
| Treatment | Gypsum application, organic matter addition, leaching | Leaching salts, improved drainage, salt-tolerant plants |
Introduction to Alkaline and Saline Soils
Alkaline soils contain high concentrations of sodium carbonate, causing their pH to exceed 8.5, which limits nutrient availability and affects plant growth. Saline soils have elevated soluble salts, primarily chlorides and sulfates of sodium, calcium, and magnesium, resulting in osmotic stress on plants. Both soil types require specific management techniques to improve soil structure and fertility for sustainable agriculture.
Key Differences Between Alkaline and Saline Soils
Alkaline soils have a high pH, typically above 8.5, due to the presence of sodium carbonate, which leads to poor nutrient availability and soil structure. Saline soils contain high concentrations of soluble salts, such as sodium chloride, causing osmotic stress that reduces water uptake by plants. The critical difference lies in their chemical composition: alkaline soils affect pH and soil alkalinity, while saline soils primarily impact salt concentration and electrical conductivity.
Causes of Soil Alkalinity
Soil alkalinity primarily results from the accumulation of sodium carbonate and bicarbonate salts, which increase the soil pH above 8.5. Common causes include poor drainage, high evaporation rates, and the use of sodium-rich irrigation water, leading to sodium dispersion in the soil profile. These conditions disrupt nutrient availability and soil structure, negatively impacting plant growth.
Factors Contributing to Soil Salinity
Soil salinity is primarily influenced by factors such as high evaporation rates, poor drainage, and the presence of soluble salts in irrigation water, which lead to the accumulation of salts on the soil surface. In alkaline soil, the dominant factor is the presence of sodium carbonate and bicarbonate, causing high pH levels and poor soil structure, whereas saline soil contains high concentrations of soluble salts like sodium chloride, sulfate, and magnesium salts without necessarily affecting pH significantly. Natural processes like parent material weathering and human activities such as excessive irrigation with salty water intensify soil salinity, impacting crop yield and soil health.
Chemical Properties of Alkaline Soils
Alkaline soils are characterized by a high pH level, typically above 8.5, due to the presence of sodium carbonate and bicarbonate compounds. These soils exhibit poor nutrient availability, particularly for micronutrients like iron, manganese, and zinc, as their solubility decreases in alkaline conditions. High sodium content causes soil dispersion, leading to poor aeration and water infiltration, which negatively affects plant growth and soil structure.
Chemical Characteristics of Saline Soils
Saline soils are characterized by high concentrations of soluble salts such as sodium chloride, calcium sulfate, and magnesium sulfate, which result in elevated electrical conductivity (EC) typically above 4 dS/m. These chemical properties cause osmotic stress limiting plant water uptake and may also disrupt nutrient availability by affecting ion exchange processes. Unlike alkaline soils, saline soils often maintain a neutral to slightly alkaline pH but have a toxic accumulation of salts that impair plant growth and soil microbial activity.
Effects on Crop Growth and Yield
Alkaline soil, marked by a high pH above 8.5, limits nutrient availability such as iron, manganese, and phosphorus, causing chlorosis and reduced crop growth, while saline soil contains high soluble salt concentrations that hinder water uptake by plants, leading to osmotic stress and toxicity. Crops grown in alkaline soils often exhibit stunted growth and lower yields due to nutrient deficiencies, whereas saline soils reduce germination rates, biomass, and overall productivity due to salt-induced physiological drought. Effective management strategies for both soil types involve proper drainage, soil amendments like gypsum for saline soils, and organic matter incorporation to improve nutrient availability and crop yield.
Management Practices for Alkaline Soils
Management practices for alkaline soils focus on lowering soil pH and improving nutrient availability through the application of gypsum (calcium sulfate) and organic matter such as compost or green manure. Incorporating sulfur-containing amendments promotes the formation of sulfuric acid, which helps neutralize excess sodium ions and reduces soil alkalinity. Proper irrigation management using high-quality water ensures leaching of sodium salts beyond the root zone, enhancing soil structure and fertility.
Reclamation Techniques for Saline Soils
Reclamation of saline soils involves leaching excess salts from the root zone using high-quality irrigation water combined with proper drainage to prevent waterlogging. Gypsum application is a common technique to replace sodium ions with calcium, improving soil structure and enhancing permeability. Incorporating organic matter and planting salt-tolerant crops also aids in restoring soil fertility and promoting microbial activity.
Conclusion: Choosing Solutions for Problem Soils
Alkaline soils, characterized by high pH levels above 8.5, often require gypsum applications and sulfur amendments to lower pH and improve nutrient availability, whereas saline soils, with high soluble salt concentrations, benefit from proper drainage and leaching to flush out excess salts. Selecting effective soil management strategies hinges on accurate soil testing to determine the specific soil chemistry and salt content. Integrating organic matter and choosing salt-tolerant crop varieties further enhances productivity in both alkaline and saline problem soils.
Alkaline soil Infographic
 libterm.com
libterm.com