Soda straws are delicate, tubular mineral formations commonly found in caves, formed by the slow deposition of calcium carbonate from dripping water. These slender, hollow structures grow vertically and are crucial indicators of cave microclimates and water chemistry. Discover how soda straws develop and their significance in understanding your natural environment by reading the rest of this article.
Table of Comparison
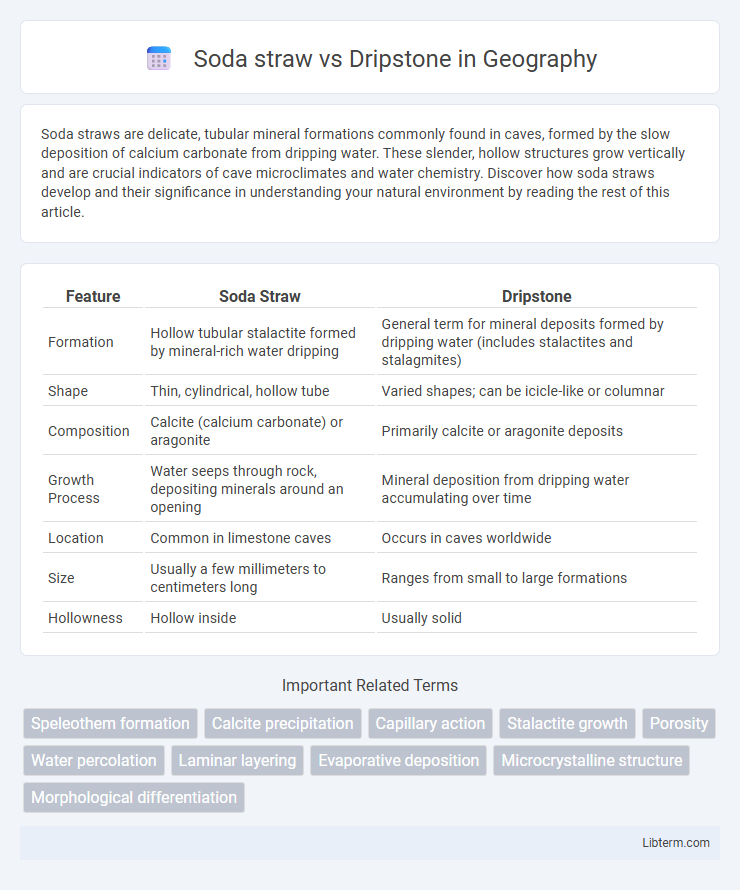
| Feature | Soda Straw | Dripstone |
|---|---|---|
| Formation | Hollow tubular stalactite formed by mineral-rich water dripping | General term for mineral deposits formed by dripping water (includes stalactites and stalagmites) |
| Shape | Thin, cylindrical, hollow tube | Varied shapes; can be icicle-like or columnar |
| Composition | Calcite (calcium carbonate) or aragonite | Primarily calcite or aragonite deposits |
| Growth Process | Water seeps through rock, depositing minerals around an opening | Mineral deposition from dripping water accumulating over time |
| Location | Common in limestone caves | Occurs in caves worldwide |
| Size | Usually a few millimeters to centimeters long | Ranges from small to large formations |
| Hollowness | Hollow inside | Usually solid |
Introduction: Understanding Soda Straws and Dripstones
Soda straws and dripstones are speleothems formed by mineral deposits in caves, primarily composed of calcium carbonate. Soda straws are thin, hollow tubes that grow downward as water deposits minerals layer by layer, while dripstones are more robust, solid formations resulting from water dripping and accumulating minerals over time. Understanding these differences helps in identifying cave formation processes and the environmental conditions influencing speleothem development.
Formation Process: How Soda Straws Develop
Soda straws develop through the slow deposition of calcium carbonate as mineral-rich water drips from cave ceilings, forming hollow tubular structures that grow downward. Unlike dripstones, which accumulate solid mass and often develop broader shapes such as stalactites or stalagmites, soda straws maintain their delicate, slender hollow form due to consistent water flow and minimal disturbance. This precise formation process highlights the unique environmental conditions necessary for soda straw growth within karst cave systems.
Formation Process: How Dripstones Evolve
Dripstones form through the gradual deposition of calcium carbonate in caves, where mineral-rich water drips from ceilings or flows along walls, creating stalactites, stalagmites, and columns over thousands of years. Soda straws represent the initial stage of dripstone formation, characterized by thin, hollow tubes resulting from slow mineral accumulation around water droplets. Over time, soda straws can thicken and solidify as water flow changes, evolving into more robust dripstones like stalactites and stalagmites through continuous mineral deposition.
Key Differences: Appearance and Structure
Soda straws are slender, hollow tubes with a smooth, cylindrical shape formed by mineral-laden water dripping slowly from cave ceilings. Dripstones, also known as stalactites or stalagmites depending on their location, are thicker, more solid formations created by mineral deposits accumulating over time from dripping water. The primary visual difference lies in soda straws' delicate, tubular structure compared to the more robust, conical shape of dripstones.
Chemical Composition Comparison
Soda straws and dripstones primarily consist of calcium carbonate (CaCO3) in the form of calcite or aragonite. Soda straws form as hollow tubular stalactites through slow mineral deposition from calcium bicarbonate-rich water, maintaining high purity with minimal impurities. Dripstones, often larger and more varied in shape, accumulate from similar mineral deposits but may incorporate additional materials like clay or organic matter, affecting their overall chemical composition.
Environmental Conditions for Formation
Soda straws form in low-turbulence environments where minimal water flow allows calcium carbonate to precipitate uniformly along thin tubular structures. Dripstones develop under more variable water flow and humidity, enabling thicker mineral deposits as water drips and evaporates on cave surfaces. Both formations require stable temperatures, high humidity, and saturated calcium bicarbonate solutions to facilitate calcite or aragonite deposition.
Growth Rates: Soda Straws vs Dripstones
Soda straws exhibit rapid growth rates, typically extending a few millimeters per year, due to their hollow, delicate tubular structure that allows water to flow through and deposit minerals efficiently. Dripstones, also known as stalactites or stalagmites, grow more slowly, often averaging around 0.13 millimeters to a few millimeters annually, as mineral deposits accumulate on their solid surfaces from dripping water. Variations in water flow rate, mineral concentration, and cave environment critically influence these differing growth rates between soda straws and dripstones.
Role in Cave Ecosystems
Soda straws and dripstones both contribute essential minerals to cave ecosystems, supporting unique microbial communities and influencing cave formations. Soda straws, thin tubular stalactites, facilitate mineral drip that forms delicate speleothems, while dripstones, which include stalactites and stalagmites, create habitats for cave-dwelling organisms by shaping microenvironments. Their slow mineral deposition processes regulate humidity and water chemistry, crucial for sustaining cave biodiversity and ecological balance.
Common Locations and Geological Settings
Soda straws commonly form in caves with slow dripping water in limestone karst environments, often found in regions like Mammoth Cave and Carlsbad Caverns. Dripstones, including stalactites and stalagmites, develop in similar limestone cave settings where calcium carbonate precipitates from dripping water, typically in humid, temperate climates. Both geological features indicate active speleothem growth in groundwater-rich cave systems with stable temperature and carbon dioxide conditions.
Significance in Speleology and Conservation
Soda straws and dripstones are critical indicators of cave formation processes and environmental conditions in speleology. Soda straws, delicate tubular formations, reveal precise drip rates and mineral saturation levels, while dripstones, formed by mineral accumulation, reflect longer-term cave stability and water chemistry. Their conservation is vital to maintaining natural speleothems, as damage disrupts mineral deposition patterns and can lead to irreversible loss of geological and ecological data.
Soda straw Infographic
 libterm.com
libterm.com