Limestone is a versatile sedimentary rock composed primarily of calcium carbonate, widely used in construction, agriculture, and manufacturing due to its durability and chemical properties. Its ability to neutralize acidic soils and serve as a raw material for cement production makes limestone essential for various industrial applications. Discover how limestone can impact your projects and why it's a key material in the article ahead.
Table of Comparison
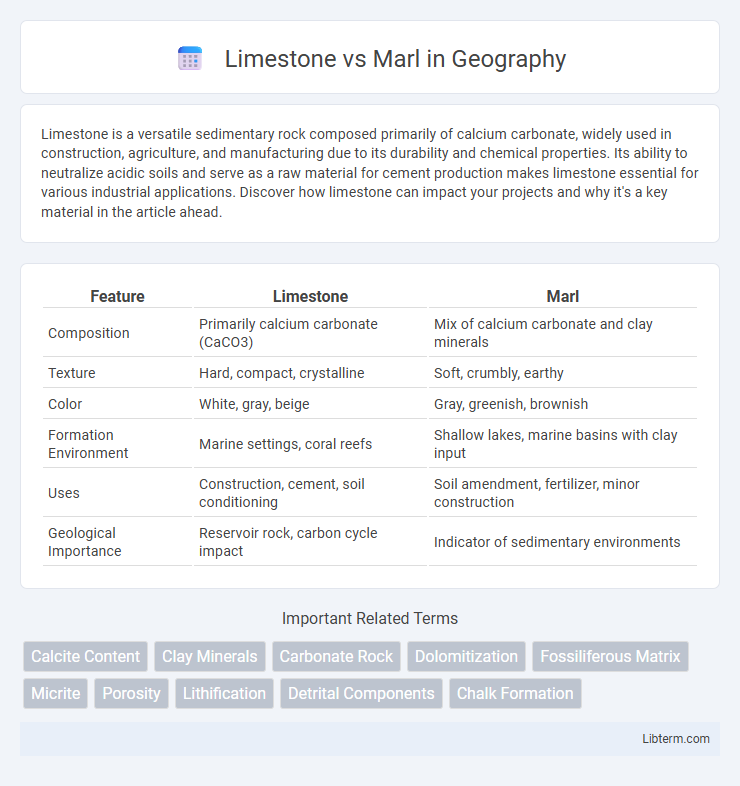
| Feature | Limestone | Marl |
|---|---|---|
| Composition | Primarily calcium carbonate (CaCO3) | Mix of calcium carbonate and clay minerals |
| Texture | Hard, compact, crystalline | Soft, crumbly, earthy |
| Color | White, gray, beige | Gray, greenish, brownish |
| Formation Environment | Marine settings, coral reefs | Shallow lakes, marine basins with clay input |
| Uses | Construction, cement, soil conditioning | Soil amendment, fertilizer, minor construction |
| Geological Importance | Reservoir rock, carbon cycle impact | Indicator of sedimentary environments |
Introduction to Limestone and Marl
Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO3), often formed from marine organisms such as coral and shell fragments. Marl is a mixed sedimentary rock or soil consisting of calcium carbonate and clay, typically formed in freshwater or marine environments with varying mineral content. Both limestone and marl play significant roles in agriculture, construction, and environmental applications due to their differing chemical compositions and physical properties.
Geological Formation of Limestone and Marl
Limestone primarily forms through the accumulation of calcium carbonate from marine organisms such as corals and shellfish in warm, shallow marine environments, resulting in a crystalline and compact texture. Marl, on the other hand, develops in freshwater or marginal marine settings where clay, silt, and calcium carbonate sediments mix, producing a softer, more argillaceous rock with higher clay content. The distinct depositional environments and sediment compositions lead to variations in texture, mineralogy, and fossil content between limestone and marl.
Chemical Composition Differences
Limestone primarily consists of calcium carbonate (CaCO3), often exceeding 90% purity, which gives it a higher concentration of calcium and carbonate ions. Marl contains a mixture of calcium carbonate, clay minerals, and varying amounts of magnesium carbonate, resulting in more complex chemical properties and lower overall CaCO3 content, typically between 35% and 65%. The presence of silicates and alumina in marl significantly alters its chemical behavior compared to the more homogenous composition of limestone.
Physical Properties Comparison
Limestone exhibits a dense, hard texture with a higher calcium carbonate content, typically above 90%, resulting in greater durability and resistance to weathering compared to marl. Marl is a softer, more porous sedimentary rock composed of a mixture of clay and calcium carbonate, usually ranging between 35-65% carbonate content, which makes it less compact and more susceptible to erosion. The color variations also differ, with limestone generally appearing in shades of white or gray, while marl often displays earthy tones such as brown, green, or yellow due to its clay minerals.
Color, Texture, and Appearance
Limestone typically exhibits a creamy to light gray color with a smooth, fine to coarse texture, often showcasing visible fossil fragments that enhance its natural aesthetic appeal. Marl, by contrast, presents a softer, muted palette ranging from pale gray to greenish or brownish hues, characterized by a more earthy, crumbly texture due to its mixed clay and carbonate composition. The appearance of limestone is generally more solid and polished, making it ideal for architectural uses, whereas marl's softer texture and varied coloration make it more suitable for agricultural and soil improvement purposes.
Common Uses in Industry and Construction
Limestone is widely used in the construction industry for producing cement, concrete, and as a building stone due to its durability and high calcium carbonate content. Marl, rich in clay and calcium carbonate, serves primarily in agriculture for soil conditioning and also acts as a raw material in cement manufacturing. Both materials are essential in industry, but limestone's structural properties make it more prominent in heavy construction projects, while marl is favored for its soil amendment benefits and moderate industrial applications.
Environmental Impact and Sustainability
Limestone extraction often results in significant habitat disruption and carbon emissions due to energy-intensive quarrying processes, whereas marl, containing a mixture of clay and carbonate, generally requires less intensive extraction, reducing its environmental footprint. Both materials play vital roles in carbon sequestration when used in agricultural and construction applications, but marl's lower carbon content limits its effectiveness compared to high-purity limestone. Sustainable management of quarry sites and the use of alternative materials like marl can mitigate environmental damage and promote long-term resource conservation.
Regional Availability and Distribution
Limestone is widely distributed across regions such as North America, Europe, and parts of Asia, forming extensive sedimentary deposits primarily in shallow marine environments. Marl, a calcium carbonate-rich mudstone with varying clay content, is commonly found in lacustrine and marine settings, with significant deposits in areas like the Mediterranean basin and parts of Central Europe. Regional availability of limestone often supports extensive quarrying for construction and industrial uses, while marl's distribution is more localized, influencing its primary use in agriculture and soil conditioning.
Strengths and Limitations of Each Material
Limestone offers high compressive strength and durability, making it ideal for structural applications and load-bearing walls, but it is susceptible to acid rain and weathering over time. Marl, composed of clay and calcium carbonate, provides good plasticity and ease of shaping, making it suitable for agricultural use and low-strength construction, but it lacks the robustness and weather resistance of limestone. Both materials vary in porosity and chemical composition, influencing their suitability for specific engineering and environmental purposes.
Choosing Between Limestone and Marl
Choosing between limestone and marl depends on soil composition and the specific crop requirements; limestone is primarily composed of calcium carbonate, providing efficient pH neutralization and improving soil structure, while marl contains varying amounts of clay and calcium carbonate, offering additional nutrients and better moisture retention. Limestone is ideal for alkaline soils requiring rapid pH adjustment, whereas marl suits acidic or sandy soils needing gradual liming and enhanced mineral content. Understanding the differences in chemical composition and soil interaction helps optimize fertilization strategies and crop yield outcomes.
Limestone Infographic
 libterm.com
libterm.com