Fetal hemoglobin (HbF) is the primary oxygen-carrying protein in a fetus, enabling efficient oxygen transfer from mother to baby. It has a higher affinity for oxygen than adult hemoglobin, which is crucial for fetal development during pregnancy. Discover how understanding fetal hemoglobin can impact your knowledge of blood disorders and treatments by reading the full article.
Table of Comparison
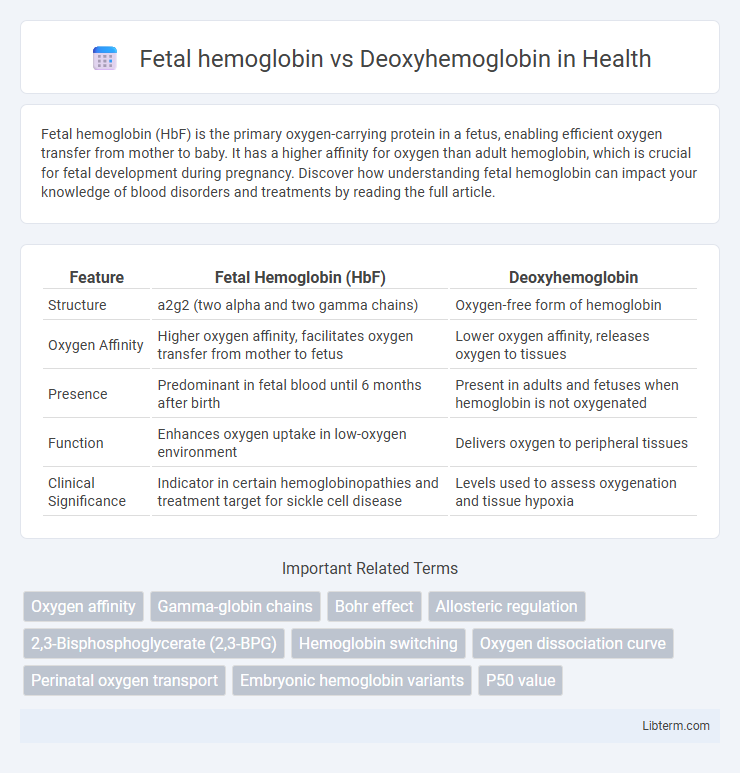
| Feature | Fetal Hemoglobin (HbF) | Deoxyhemoglobin |
|---|---|---|
| Structure | a2g2 (two alpha and two gamma chains) | Oxygen-free form of hemoglobin |
| Oxygen Affinity | Higher oxygen affinity, facilitates oxygen transfer from mother to fetus | Lower oxygen affinity, releases oxygen to tissues |
| Presence | Predominant in fetal blood until 6 months after birth | Present in adults and fetuses when hemoglobin is not oxygenated |
| Function | Enhances oxygen uptake in low-oxygen environment | Delivers oxygen to peripheral tissues |
| Clinical Significance | Indicator in certain hemoglobinopathies and treatment target for sickle cell disease | Levels used to assess oxygenation and tissue hypoxia |
Introduction to Hemoglobin Types
Fetal hemoglobin (HbF) differs from deoxyhemoglobin, a form of adult hemoglobin (HbA), in structure and oxygen affinity, with HbF containing two alpha and two gamma chains compared to the alpha and beta chains of HbA. HbF has a higher oxygen-binding capacity, facilitating efficient oxygen transfer from maternal to fetal blood during pregnancy. Deoxyhemoglobin refers to hemoglobin molecules not bound to oxygen, critical for oxygen delivery to tissues, whereas HbF dominates in the fetal bloodstream before gradually being replaced by HbA after birth.
Structure and Function of Fetal Hemoglobin
Fetal hemoglobin (HbF) consists of two alpha and two gamma globin chains, differing structurally from adult hemoglobin (HbA) which contains beta chains, enabling higher oxygen affinity essential for efficient oxygen transfer from the maternal bloodstream to the fetus. Its structurally distinct gamma chains reduce binding to 2,3-Bisphosphoglycerate (2,3-BPG), increasing oxygen affinity compared to deoxyhemoglobin in adults, which facilitates oxygen uptake under low oxygen tension in the placenta. Functionally, HbF's enhanced oxygen-binding capacity supports fetal development by optimizing oxygen delivery during prenatal life, contrasting with deoxyhemoglobin's role in oxygen release to tissues.
Structure and Characteristics of Deoxyhemoglobin
Deoxyhemoglobin is the form of hemoglobin without bound oxygen, characterized by a tense (T) quaternary structure that results in decreased affinity for oxygen compared to fetal hemoglobin. Its structure promotes the release of oxygen to tissues by stabilizing salt bridges and hydrogen bonds in the beta subunits, unlike fetal hemoglobin which has a relaxed (R) state due to higher affinity. The unique alpha2gamma2 composition of fetal hemoglobin allows it to bind oxygen more tightly, facilitating efficient oxygen transfer from maternal to fetal blood.
Oxygen Affinity: Fetal vs Deoxyhemoglobin
Fetal hemoglobin (HbF) exhibits a higher oxygen affinity compared to deoxyhemoglobin (Hb) due to its unique subunit composition, allowing efficient oxygen transfer from maternal blood. The gamma chains in HbF reduce its interaction with 2,3-bisphosphoglycerate (2,3-BPG), stabilizing the oxygen-bound form and promoting oxygen uptake at lower partial pressures. In contrast, deoxyhemoglobin's lower oxygen affinity facilitates oxygen release to tissues, adapting to physiological oxygen demands.
Role in Fetal Development
Fetal hemoglobin (HbF) has a higher affinity for oxygen than deoxyhemoglobin, enabling efficient oxygen transfer from the maternal blood to the fetus across the placenta. HbF consists of two alpha and two gamma chains, which enhance oxygen binding under low oxygen tension conditions typical of the fetal environment. The predominance of fetal hemoglobin during gestation is crucial for sustaining adequate oxygen delivery to rapidly growing fetal tissues before the switch to adult hemoglobin (HbA) after birth.
Physiological Significance of Deoxyhemoglobin
Deoxyhemoglobin plays a crucial physiological role by facilitating oxygen release from red blood cells to tissues, enabling efficient cellular respiration under varying oxygen demands. Its lower affinity for oxygen compared to fetal hemoglobin ensures that oxygen is readily delivered to fetal tissues while maintaining higher oxygen binding in the fetal bloodstream. The dynamic equilibrium between deoxyhemoglobin and oxyhemoglobin is essential for regulating blood oxygen transport, responding adaptively to hypoxic conditions.
Genetic Regulation of Hemoglobin Variants
Fetal hemoglobin (HbF) expression is predominantly regulated by the gamma-globin gene promoters influenced by transcription factors such as BCL11A, KLF1, and LRF, which repress gamma-globin expression postnatally, facilitating the switch to adult hemoglobin (HbA) composed of alpha and beta chains. Deoxyhemoglobin refers to the oxygen-depleted form of hemoglobin, primarily adult hemoglobin variants governed by beta-globin gene regulation rather than the gamma-globin genes associated with HbF. Genetic mutations or polymorphisms affecting regulatory elements, including the locus control region (LCR) and promoter regions, can modulate the hemoglobin switching mechanism and influence the persistence of HbF in adults, impacting conditions like sickle cell disease and beta-thalassemia.
Clinical Implications and Disorders
Fetal hemoglobin (HbF) exhibits higher oxygen affinity than deoxyhemoglobin (Hb) due to its unique g-globin chains, facilitating efficient oxygen transfer across the placenta. Clinically, elevated HbF levels can ameliorate symptoms in hemoglobinopathies like sickle cell disease by inhibiting HbS polymerization, whereas deoxyhemoglobin accumulation under hypoxic conditions contributes to tissue ischemia and vaso-occlusive crises. Disorders such as hereditary persistence of fetal hemoglobin (HPFH) result in sustained HbF expression, providing therapeutic benefit in b-thalassemia and sickle cell anemia by improving oxygen delivery and reducing hemolysis.
Diagnostic Differences: Biomarkers and Measurement
Fetal hemoglobin (HbF) is primarily measured using high-performance liquid chromatography (HPLC) or electrophoresis, serving as a biomarker to diagnose hemoglobinopathies such as beta-thalassemia and sickle cell disease. Deoxyhemoglobin, representing the oxygen-unbound form of adult hemoglobin (HbA), is commonly quantified through pulse oximetry and co-oximetry to assess tissue oxygenation and conditions like hypoxemia. The distinct spectral properties and oxygen-binding affinities enable differential detection techniques, critical for accurate diagnosis and monitoring in clinical hematology and neonatology.
Therapeutic Perspectives and Future Directions
Fetal hemoglobin (HbF) demonstrates higher oxygen affinity than deoxyhemoglobin (Hb), making it a critical target in treating hemoglobinopathies like sickle cell disease and beta-thalassemia by reactivating HbF production to improve oxygen delivery. Therapeutic strategies involving pharmacological agents such as hydroxyurea and gene-editing technologies aim to upregulate HbF synthesis, reducing sickling and enhancing red blood cell function. Future directions include CRISPR-based gene therapies and novel fetal hemoglobin inducers designed to provide sustained HbF expression, offering promising avenues for durable disease amelioration.
Fetal hemoglobin Infographic
 libterm.com
libterm.com