Uncompetitive markets hinder innovation and limit consumer choices, resulting in higher prices and reduced quality. Businesses struggling to stay relevant must adapt quickly to avoid losing their competitive edge. Explore the rest of this article to understand how you can thrive in today's challenging economic landscape.
Table of Comparison
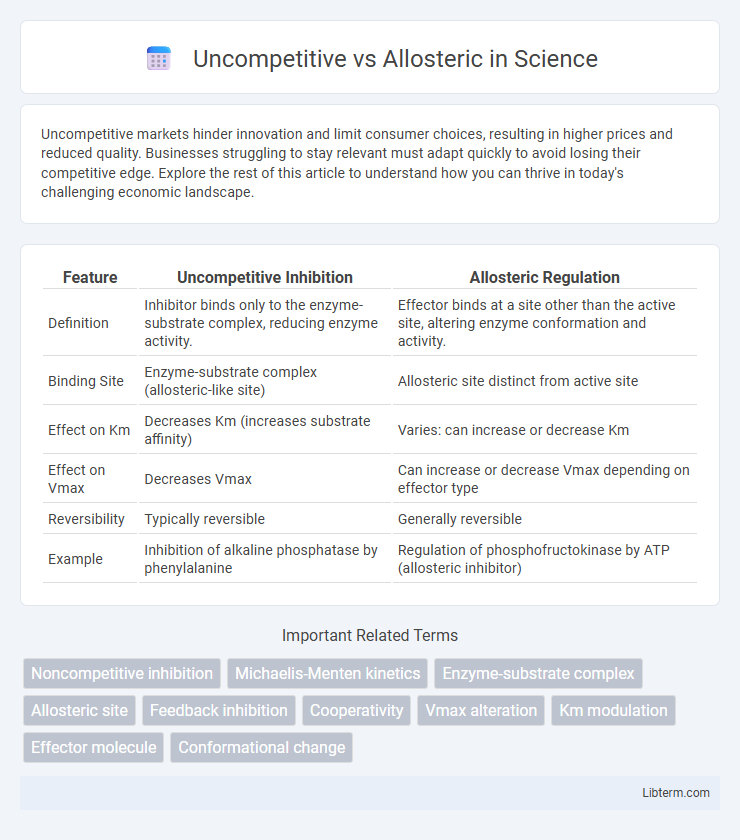
| Feature | Uncompetitive Inhibition | Allosteric Regulation |
|---|---|---|
| Definition | Inhibitor binds only to the enzyme-substrate complex, reducing enzyme activity. | Effector binds at a site other than the active site, altering enzyme conformation and activity. |
| Binding Site | Enzyme-substrate complex (allosteric-like site) | Allosteric site distinct from active site |
| Effect on Km | Decreases Km (increases substrate affinity) | Varies: can increase or decrease Km |
| Effect on Vmax | Decreases Vmax | Can increase or decrease Vmax depending on effector type |
| Reversibility | Typically reversible | Generally reversible |
| Example | Inhibition of alkaline phosphatase by phenylalanine | Regulation of phosphofructokinase by ATP (allosteric inhibitor) |
Introduction to Enzyme Inhibition
Uncompetitive inhibition occurs when an inhibitor binds exclusively to the enzyme-substrate complex, reducing both the maximum reaction rate (Vmax) and the Michaelis constant (Km). Allosteric inhibition involves the binding of a molecule at a site distinct from the active site, causing conformational changes that decrease enzyme activity without directly competing with the substrate. Both mechanisms play crucial roles in regulating enzyme function and are essential in drug design targeting specific enzymatic pathways.
Overview of Uncompetitive Inhibition
Uncompetitive inhibition occurs when an inhibitor binds exclusively to the enzyme-substrate complex, decreasing both the apparent Vmax and Km values. This type of inhibition differs from allosteric inhibition, where the regulator binds to an allosteric site regardless of substrate presence, modulating enzyme activity by inducing conformational changes. Uncompetitive inhibitors are often studied in enzyme kinetics to understand their unique mechanism and therapeutic potential in targeting multi-substrate enzymes.
Mechanism of Uncompetitive Inhibitors
Uncompetitive inhibitors bind exclusively to the enzyme-substrate complex, preventing the complex from converting into product, which decreases both the apparent Km and Vmax. This binding site is distinct from the active site, often inducing conformational changes that stabilize the enzyme-substrate-inhibitor complex and hinder catalytic activity. The mechanism contrasts with allosteric inhibitors, which typically bind to regulatory sites and alter enzyme activity through conformational shifts without requiring substrate presence.
Allosteric Inhibition Defined
Allosteric inhibition occurs when an inhibitor binds to a site on the enzyme distinct from the active site, causing a conformational change that reduces enzyme activity. This type of inhibition differs from uncompetitive inhibition, which requires the substrate to be bound first and the inhibitor binding exclusively to the enzyme-substrate complex. Allosteric inhibitors regulate enzyme function by modulating the enzyme's shape and dynamics, often resulting in reversible and fine-tuned control of metabolic pathways.
Mechanism of Allosteric Inhibitors
Allosteric inhibitors bind to a site distinct from the active site on an enzyme, causing a conformational change that reduces its catalytic activity without directly competing with the substrate. This mechanism contrasts with uncompetitive inhibition, where the inhibitor binds only to the enzyme-substrate complex, stabilizing it and preventing product formation. The allosteric modulation specifically alters enzyme dynamics, offering potential for selective regulation of enzyme function in metabolic pathways.
Key Differences Between Uncompetitive and Allosteric Inhibition
Uncompetitive inhibition occurs when an inhibitor binds exclusively to the enzyme-substrate complex, decreasing both the maximum reaction rate (Vmax) and the apparent affinity (Km) by stabilizing the complex. Allosteric inhibition involves the binding of an inhibitor to a regulatory site other than the active site, causing conformational changes that reduce enzyme activity without directly competing with the substrate. Key differences include the binding site specificity--uncompetitive inhibitors bind only post-substrate binding, while allosteric inhibitors target distinct regulatory sites--and their impact on enzyme kinetics, where uncompetitive inhibition affects both Vmax and Km, whereas allosteric inhibition often leads to non-Michaelis-Menten behavior and modulates enzyme activity through structural alterations.
Effects on Enzyme Kinetics
Uncompetitive inhibition decreases both the apparent maximum velocity (Vmax) and Michaelis constant (Km) by binding exclusively to the enzyme-substrate complex, thereby stabilizing it and reducing the rate of product formation. Allosteric inhibition involves binding to a site other than the active site, causing conformational changes that alter the enzyme's activity, which can either decrease Vmax without changing Km or modify substrate affinity depending on the allosteric mechanism. The distinct kinetic effects of uncompetitive versus allosteric inhibitors are critical for understanding enzyme regulation and designing targeted pharmaceuticals.
Biological Examples and Significance
Uncompetitive inhibition occurs when an inhibitor binds exclusively to the enzyme-substrate complex, exemplified by the inhibition of alkaline phosphatase by L-phenylalanine, which stabilizes the complex and decreases enzyme activity. Allosteric inhibition involves inhibitor binding to a site distinct from the active site, altering enzyme conformation and activity, as seen in the regulation of phosphofructokinase by ATP in glycolysis. These mechanisms are biologically significant for fine-tuning metabolic pathways, ensuring precise control over enzymatic reactions in response to cellular conditions.
Therapeutic Applications and Drug Design
Uncompetitive inhibitors bind only to the enzyme-substrate complex, enhancing specificity and reducing off-target effects, making them valuable in treatments for diseases like Alzheimer's and cancer where precise enzyme modulation is critical. Allosteric inhibitors interact at sites distinct from the active site, offering versatile therapeutic applications by modulating enzyme activity without directly competing with the substrate, which is beneficial in drug design for conditions such as metabolic disorders and infectious diseases. Both uncompetitive and allosteric mechanisms provide innovative strategies in drug development, enabling the creation of selective, potent therapeutics with improved safety profiles.
Conclusion: Choosing the Right Inhibition Strategy
Uncompetitive inhibition is most effective when substrate concentration is high, as it binds only to the enzyme-substrate complex, reducing enzyme activity without affecting substrate binding. Allosteric inhibition offers versatile regulation by binding to sites other than the active site, enabling fine-tuned control of enzyme function through conformational changes. Selecting the appropriate inhibition strategy depends on the enzyme mechanism, desired control level, and metabolic context, with uncompetitive inhibitors suited for pathways with accumulated substrates and allosteric inhibitors optimal for dynamic, feedback-regulated processes.
Uncompetitive Infographic
 libterm.com
libterm.com