Competitive inhibition occurs when a molecule similar to the substrate competes for binding at the active site of an enzyme, reducing its activity. This process directly impacts enzymatic reactions by preventing substrate molecules from accessing the active site, leading to a decrease in the reaction rate. Discover how competitive inhibition influences biochemical pathways and its significance in drug design by reading the rest of the article.
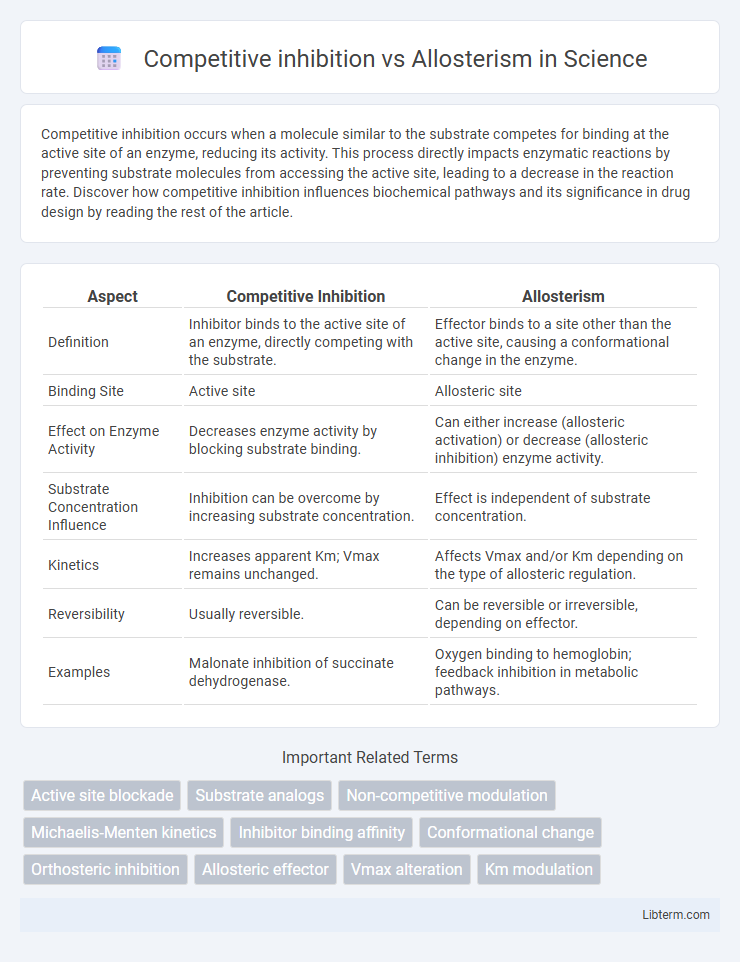
Table of Comparison
| Aspect | Competitive Inhibition | Allosterism |
|---|---|---|
| Definition | Inhibitor binds to the active site of an enzyme, directly competing with the substrate. | Effector binds to a site other than the active site, causing a conformational change in the enzyme. |
| Binding Site | Active site | Allosteric site |
| Effect on Enzyme Activity | Decreases enzyme activity by blocking substrate binding. | Can either increase (allosteric activation) or decrease (allosteric inhibition) enzyme activity. |
| Substrate Concentration Influence | Inhibition can be overcome by increasing substrate concentration. | Effect is independent of substrate concentration. |
| Kinetics | Increases apparent Km; Vmax remains unchanged. | Affects Vmax and/or Km depending on the type of allosteric regulation. |
| Reversibility | Usually reversible. | Can be reversible or irreversible, depending on effector. |
| Examples | Malonate inhibition of succinate dehydrogenase. | Oxygen binding to hemoglobin; feedback inhibition in metabolic pathways. |
Introduction to Enzyme Regulation
Competitive inhibition occurs when a molecule similar to the substrate binds to the enzyme's active site, preventing substrate attachment and decreasing enzyme activity. Allosterism involves the binding of regulatory molecules at distinct allosteric sites, inducing conformational changes that modulate enzyme function either by enhancing or inhibiting activity. These mechanisms are crucial for enzyme regulation, allowing cells to fine-tune metabolic pathways in response to changing physiological conditions.
Defining Competitive Inhibition
Competitive inhibition occurs when an inhibitor molecule binds directly to the active site of an enzyme, competing with the substrate for the same binding location. This type of inhibition increases the apparent Km of the enzyme without affecting the maximum velocity (Vmax), as the inhibitor can be outcompeted by a high substrate concentration. In contrast, allosterism involves the binding of an effector molecule to a site other than the active site, causing conformational changes that modulate enzyme activity either positively or negatively.
Exploring Allosterism in Enzyme Activity
Allosterism in enzyme activity involves the binding of regulatory molecules at sites distinct from the active site, causing conformational changes that alter enzyme function. Unlike competitive inhibition, which directly blocks the active site by competing with the substrate, allosteric regulation can either enhance or inhibit enzyme activity by modifying the enzyme's shape and dynamics. This mechanism allows for finely tuned metabolic control, exemplified by enzymes such as aspartate transcarbamoylase and hemoglobin, where allosteric effectors modulate catalytic efficiency and substrate affinity.
Mechanisms of Competitive Inhibition
Competitive inhibition occurs when an inhibitor molecule structurally resembles the substrate and binds directly to the enzyme's active site, blocking substrate access and preventing catalysis. This reversible binding increases the apparent Km without affecting the maximum reaction velocity (Vmax), as the substrate can outcompete the inhibitor at high concentrations. The mechanism relies on the precise affinity between the inhibitor and the active site, which is critical for modulating enzyme activity in metabolic pathways and drug design.
Mechanisms of Allosteric Regulation
Allosteric regulation involves the binding of effector molecules to specific sites distinct from the active site, causing conformational changes that modulate enzyme activity. This mechanism alters the enzyme's shape and dynamics, either enhancing (positive allosterism) or inhibiting (negative allosterism) substrate affinity and catalytic efficiency. Unlike competitive inhibition, which directly blocks the active site by substrate analogs, allosteric regulation provides a more versatile, fine-tuned control over enzyme function essential for complex metabolic pathways.
Structural Differences: Competitive vs Allosteric Sites
Competitive inhibition occurs when inhibitors bind directly to the active site of an enzyme, structurally resembling the substrate to block substrate access. In contrast, allosterism involves molecules binding to distinct allosteric sites separate from the active site, inducing conformational changes that modulate enzyme activity. The structural differences between competitive and allosteric sites are key to their respective regulatory mechanisms, with competitive sites being substrate-specific and allosteric sites enabling broader functional modulation.
Impact on Enzyme Kinetics
Competitive inhibition increases the apparent Km of an enzyme without affecting Vmax, as the inhibitor competes with the substrate for the active site, reducing substrate binding efficiency. Allosteric modulation alters enzyme kinetics by binding to a site distinct from the active site, causing conformational changes that can either enhance or inhibit enzyme activity, thereby impacting both Km and Vmax depending on the nature of the effector. This differential regulation influences the enzyme's response to substrate concentration, making allosterism a versatile mechanism for controlling metabolic pathways.
Examples in Biological Systems
Competitive inhibition occurs when a molecule structurally resembles the substrate and binds directly to the active site of enzymes like hexokinase in glucose metabolism, blocking substrate access. Allosterism involves effectors binding to sites other than the active site, inducing conformational changes that modulate enzyme activity, exemplified by oxygen binding to hemoglobin altering its affinity through cooperative interactions. Both mechanisms regulate enzyme function but differ in their interaction sites and impacts on enzyme kinetics.
Pharmacological Implications
Competitive inhibition involves a drug binding to the active site of an enzyme, directly blocking substrate interaction, which allows for reversible modulation of enzyme activity and dose-dependent effects useful in managing conditions like hypertension. Allosterism entails a drug binding to a distinct regulatory site, causing conformational changes that alter enzyme or receptor activity, offering greater specificity and potential for fine-tuned therapeutic responses with less risk of complete inhibition. Pharmacological implications include competitive inhibitors requiring careful dosing to avoid toxicity, while allosteric modulators can enhance or diminish endogenous ligand effects, expanding treatment possibilities for diseases such as cancer and neurological disorders.
Summary: Key Differences between Competitive Inhibition and Allosterism
Competitive inhibition involves an inhibitor binding directly to the enzyme's active site, blocking substrate access, whereas allosterism involves effector molecules binding to separate allosteric sites, inducing conformational changes that alter enzyme activity. Competitive inhibition is typically reversible and affects substrate affinity (Km) without changing maximum velocity (Vmax), while allosterism can modulate both Km and Vmax through positive or negative regulation. These mechanisms differ fundamentally in binding sites, effects on enzyme kinetics, and regulatory roles within metabolic pathways.
Competitive inhibition Infographic
 libterm.com
libterm.com