Concentrated formulas deliver higher potency by minimizing fillers and maximizing active ingredients for superior effectiveness. This approach ensures you get the most benefit from each dose, enhancing performance and results. Explore the rest of this article to discover how concentrated products can transform your routine.
Table of Comparison
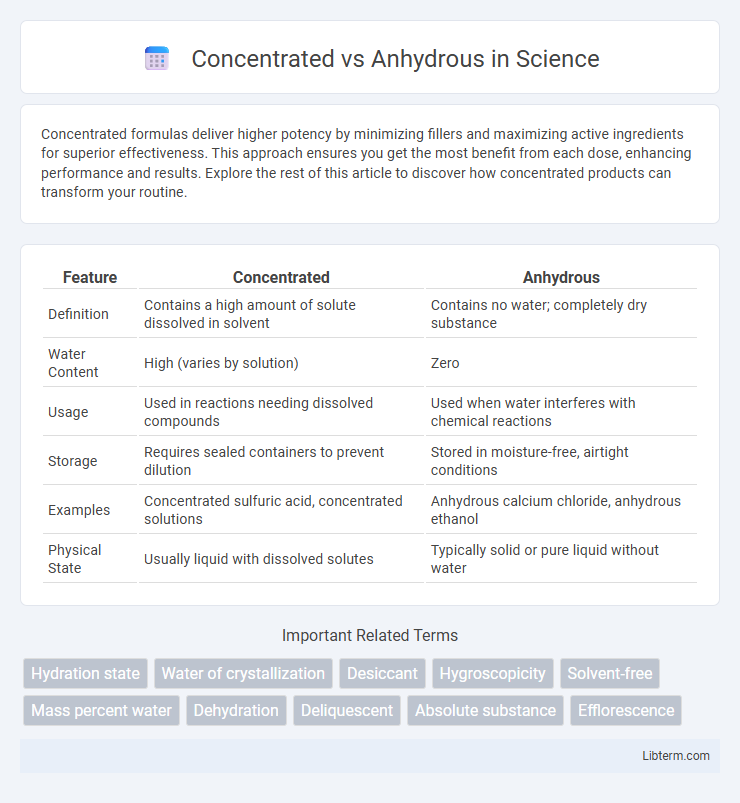
| Feature | Concentrated | Anhydrous |
|---|---|---|
| Definition | Contains a high amount of solute dissolved in solvent | Contains no water; completely dry substance |
| Water Content | High (varies by solution) | Zero |
| Usage | Used in reactions needing dissolved compounds | Used when water interferes with chemical reactions |
| Storage | Requires sealed containers to prevent dilution | Stored in moisture-free, airtight conditions |
| Examples | Concentrated sulfuric acid, concentrated solutions | Anhydrous calcium chloride, anhydrous ethanol |
| Physical State | Usually liquid with dissolved solutes | Typically solid or pure liquid without water |
Understanding the Terms: Concentrated vs Anhydrous
Concentrated substances have a high proportion of a solute dissolved in a solvent, increasing the solution's potency or strength, while anhydrous compounds completely lack water molecules, making them ideal for reactions sensitive to moisture. Understanding the difference between concentrated and anhydrous forms is essential in chemistry for selecting the correct form in processes such as synthesis, drying, or storage. Concentrated solutions often contain water or another solvent, whereas anhydrous chemicals are dry and require careful handling to prevent moisture absorption.
Key Differences Between Concentrated and Anhydrous Substances
Concentrated substances contain a high amount of solute dissolved in a solvent, resulting in a dense solution with specific concentration levels, whereas anhydrous substances are completely devoid of water molecules in their chemical structure. The presence of water significantly affects physical properties such as melting point and reactivity, making anhydrous compounds more stable in certain chemical reactions. Applications vary widely; concentrated solutions are commonly used in industrial and laboratory processes requiring precise solute measurements, while anhydrous materials are critical in moisture-sensitive reactions and manufacturing.
Chemical Composition: What Sets Them Apart?
Concentrated solutions contain a high proportion of solute dissolved in a solvent, resulting in a specific mixture where chemical interactions between components influence properties such as boiling point and reactivity. Anhydrous substances, on the other hand, lack any water molecules, representing a pure chemical state that significantly alters their physical and chemical behaviors compared to their hydrated or concentrated counterparts. The absence of water in anhydrous compounds often leads to increased stability and different reactivity patterns critical in industrial and laboratory applications.
Common Uses of Concentrated Solutions
Concentrated solutions are widely used in chemical laboratories for titrations and industrial processes, where precise reagent volumes are critical for reactions such as acid-base neutralizations and synthesis of pharmaceuticals. Common applications include concentrated sulfuric acid for dehydration reactions, concentrated hydrochloric acid for metal cleaning, and concentrated sodium hydroxide for saponification. These solutions enable efficient handling and storage of chemicals with high reactivity and density, reducing transportation costs and improving reaction control.
Applications of Anhydrous Materials
Anhydrous materials are widely used in applications requiring the absence of moisture, such as in pharmaceuticals, catalysts, and chemical synthesis to ensure stability and reactivity. In industries like aerospace and electronics, anhydrous compounds prevent corrosion and moisture-induced degradation, enhancing product longevity and performance. Their moisture-free nature makes them essential in processes like drying agents, dehydrated solvents, and battery manufacturing where water presence can be detrimental.
Storage and Handling Considerations
Concentrated chemicals require specialized storage containers resistant to corrosion and tightly sealed to prevent vapor release and contamination. Anhydrous substances demand moisture-free environments with desiccants or inert gas blankets to avoid hydrolysis and degradation. Both types mandate temperature-controlled areas away from incompatible materials to ensure stability and safety during handling.
Safety Precautions: Risks and Hazards
Concentrated and anhydrous chemicals pose distinct safety risks due to their high reactivity and potential for severe chemical burns or inhalation hazards. Proper handling requires the use of personal protective equipment such as gloves, goggles, and respiratory protection to prevent exposure to toxic fumes and corrosive splashes. Storage in well-ventilated, temperature-controlled environments minimizes the risk of accidental spills, fires, or chemical reactions associated with both concentrated and anhydrous substances.
Industrial Relevance in Various Sectors
Concentrated and anhydrous chemicals play crucial roles in industrial applications, with concentrated solutions favored for ease of handling and precise dilution in sectors like pharmaceuticals and food processing. Anhydrous compounds are essential in industries requiring moisture-free conditions, such as electronics manufacturing, petrochemicals, and specialty chemical production. Their industrial relevance is defined by stability, purity, and specific process requirements, influencing product formulation and operational efficiency.
Choosing the Right Option: Factors to Consider
Choosing between concentrated and anhydrous forms depends on factors such as application purpose, formulation compatibility, and desired intensity of active ingredients. Concentrated products often offer ease of dilution and controlled dosing, making them ideal for large-scale or adjustable uses, while anhydrous forms provide stability and longer shelf life due to the absence of water. Consider storage conditions, target efficacy, and potential interactions when selecting the optimal form for industrial, cosmetic, or chemical applications.
Summary Table: Concentrated vs Anhydrous at a Glance
The Summary Table: Concentrated vs Anhydrous at a Glance highlights key differences in chemical composition, water content, and applications. Concentrated substances contain higher water percentages, offering easier handling but lower purity, while anhydrous forms are water-free, providing higher purity and reactivity. Typical uses include concentrated solutions in industrial processes and anhydrous compounds in laboratory reactions requiring minimal moisture interference.
Concentrated Infographic
 libterm.com
libterm.com