Structural isomers are compounds with the same molecular formula but different physical and chemical properties due to the varied connectivity of their atoms. These variations can drastically affect boiling points, melting points, and reactivity, making them crucial in chemistry and pharmaceuticals. To understand how structural isomers impact your studies or work, continue reading the rest of the article.
Table of Comparison
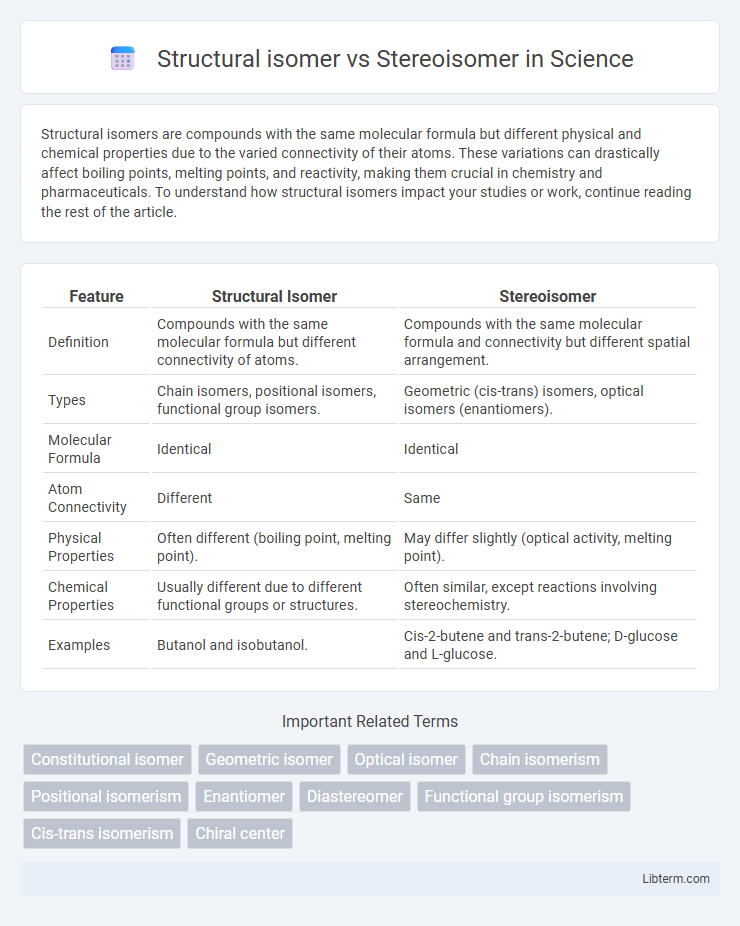
| Feature | Structural Isomer | Stereoisomer |
|---|---|---|
| Definition | Compounds with the same molecular formula but different connectivity of atoms. | Compounds with the same molecular formula and connectivity but different spatial arrangement. |
| Types | Chain isomers, positional isomers, functional group isomers. | Geometric (cis-trans) isomers, optical isomers (enantiomers). |
| Molecular Formula | Identical | Identical |
| Atom Connectivity | Different | Same |
| Physical Properties | Often different (boiling point, melting point). | May differ slightly (optical activity, melting point). |
| Chemical Properties | Usually different due to different functional groups or structures. | Often similar, except reactions involving stereochemistry. |
| Examples | Butanol and isobutanol. | Cis-2-butene and trans-2-butene; D-glucose and L-glucose. |
Introduction to Isomerism
Isomerism encompasses the phenomenon where compounds share the same molecular formula but differ in structural or spatial arrangement, leading to distinct properties. Structural isomers differ in the connectivity of atoms, resulting in variations such as chain, positional, or functional group isomers. Stereoisomers maintain the same atomic connectivity but vary in the three-dimensional orientation of atoms, including enantiomers and diastereomers, crucial in biochemical specificity and pharmaceutical applications.
Defining Structural Isomers
Structural isomers are compounds with the same molecular formula but different connectivity of atoms, resulting in distinct structures and properties. These isomers include chain isomers, positional isomers, and functional group isomers, each varying in the arrangement of carbon skeletons, attachment points, or functional groups. Unlike stereoisomers, which differ in spatial orientation, structural isomers differ in the actual bonding patterns within the molecule.
Key Types of Structural Isomers
Structural isomers differ in the connectivity of atoms within molecules, leading to variations such as chain isomers, where carbon chain arrangements vary, positional isomers, with functional groups attached at different positions, and functional group isomers, which have distinct functional groups altogether. These variations result in unique physical and chemical properties despite having the same molecular formula. In contrast, stereoisomers share the same atom connectivity but differ in spatial arrangement, including enantiomers and diastereomers.
Defining Stereoisomers
Stereoisomers are molecules with the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. Unlike structural isomers, which differ in the connectivity of their atoms, stereoisomers maintain connectivity but vary in spatial arrangement. Key types of stereoisomers include enantiomers and diastereomers, crucial in fields like pharmaceuticals due to their distinct chemical properties.
Main Types of Stereoisomers
Structural isomers differ in the connectivity of atoms, whereas stereoisomers share the same atomic connectivity but differ in spatial arrangement. The main types of stereoisomers include enantiomers, which are non-superimposable mirror images, and diastereomers, which are stereoisomers not related as mirror images. Geometric isomers, a subtype of diastereomers, arise from restricted rotation around double bonds or rings, resulting in distinct cis and trans configurations.
Structural Isomers vs Stereoisomers: Core Differences
Structural isomers differ in the connectivity of atoms, resulting in variations such as chain, positional, and functional group isomers, while stereoisomers have the same atomic sequence but differ in spatial arrangement, including enantiomers and diastereomers. Structural isomers exhibit distinct physical and chemical properties due to different bonding frameworks, whereas stereoisomers share many properties but differ in optical activity and spatial interactions. Understanding these core differences is crucial for applications in organic synthesis, pharmacology, and materials science.
Importance in Organic Chemistry
Structural isomers and stereoisomers play crucial roles in organic chemistry by influencing molecular properties and reactivity. Structural isomers differ in the connectivity of atoms, affecting physical and chemical behavior, while stereoisomers share the same connectivity but differ in spatial arrangement, impacting biological activity and stereospecific reactions. Understanding these isomers is essential for drug design, synthesis, and predicting molecular interactions.
Identifying Isomers: Methods and Techniques
Identifying structural isomers involves techniques such as mass spectrometry and infrared spectroscopy, which differentiate isomers based on variations in molecular connectivity and functional groups. Stereoisomers require methods like nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography to analyze spatial arrangements around chiral centers or double bonds. Chromatographic techniques, including gas chromatography (GC) and high-performance liquid chromatography (HPLC), separate isomers by exploiting differences in physical properties.
Real-World Examples and Applications
Structural isomers differ in the connectivity of their atoms, as seen in compounds like butane and isobutane, where variations affect fuel combustion properties. Stereoisomers, such as enantiomers of the drug thalidomide, showcase how 3D spatial arrangement influences biological activity and safety. These differences are critical in pharmaceuticals, where structural isomers can alter drug metabolism, while stereoisomers determine drug efficacy and side effects.
Summary and Conclusion
Structural isomers differ in the connectivity of atoms within a molecule, resulting in distinct bonding patterns and molecular frameworks, while stereoisomers share the same atom-to-atom connections but differ in the spatial arrangement of atoms, impacting properties like optical activity and reactivity. Structural isomers include chain, position, and functional group isomers, whereas stereoisomers consist of enantiomers and diastereomers, vital for understanding molecular behavior in organic chemistry and pharmaceuticals. Recognizing the differences between structural and stereoisomers is crucial for chemical identification, synthesis, and application in fields ranging from drug design to material science.
Structural isomer Infographic
 libterm.com
libterm.com