Crystal habit refers to the characteristic external shape displayed by individual crystals or aggregates, dictated by their internal atomic arrangement and environmental conditions during formation. Understanding crystal habit helps you identify minerals and influences their practical applications in industries such as jewelry and electronics. Explore the rest of the article to discover how crystal habit impacts mineral properties and classification.
Table of Comparison
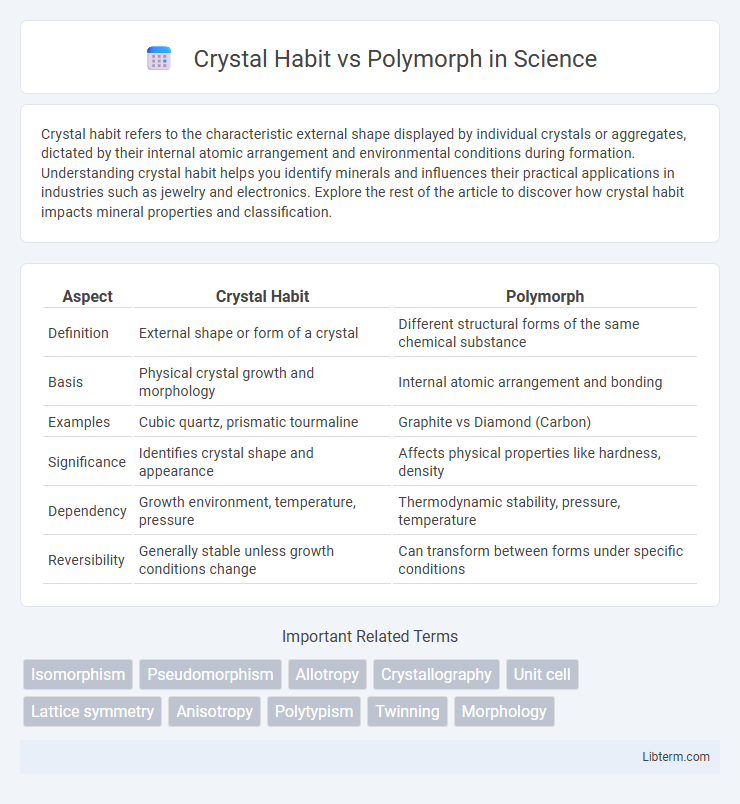
| Aspect | Crystal Habit | Polymorph |
|---|---|---|
| Definition | External shape or form of a crystal | Different structural forms of the same chemical substance |
| Basis | Physical crystal growth and morphology | Internal atomic arrangement and bonding |
| Examples | Cubic quartz, prismatic tourmaline | Graphite vs Diamond (Carbon) |
| Significance | Identifies crystal shape and appearance | Affects physical properties like hardness, density |
| Dependency | Growth environment, temperature, pressure | Thermodynamic stability, pressure, temperature |
| Reversibility | Generally stable unless growth conditions change | Can transform between forms under specific conditions |
Introduction to Crystal Habit and Polymorph
Crystal habit refers to the external shape displayed by a crystal, determined by its internal atomic structure and growth conditions, which can vary widely even among the same mineral. Polymorphs are different crystal structures of the same chemical substance, exhibiting distinct physical properties and crystal habits due to variations in atomic arrangement. Understanding the relationship between crystal habit and polymorphism is essential for identifying minerals and tailoring materials in fields like geology and materials science.
Defining Crystal Habit
Crystal habit defines the external shape or form that individual crystals exhibit during growth, determined by the underlying crystal structure and environmental conditions. It contrasts with polymorphism, which refers to the ability of a substance to exist in more than one crystal structure or form. Understanding crystal habit is crucial for identifying minerals, as it influences properties like cleavage and fracture patterns independent of polymorphic variation.
Understanding Polymorphism in Minerals
Polymorphism in minerals occurs when a single chemical composition crystallizes into different structural forms, known as polymorphs, each exhibiting distinct physical properties. Crystal habit refers to the external shape or morphology a mineral naturally forms, influenced by environmental conditions during growth, but does not change the mineral's internal structure. Understanding polymorphism is crucial for identifying minerals accurately, as polymorphs like graphite and diamond share the same carbon composition yet differ drastically in crystal structure and hardness.
Key Differences Between Crystal Habit and Polymorph
Crystal habit refers to the characteristic external shape displayed by individual crystals of a mineral, determined by the internal arrangement of atoms and environmental growth conditions. Polymorphs are distinct minerals that share the same chemical composition but differ in crystal structure due to variations in atomic arrangement, resulting in different physical properties. The key difference lies in crystal habit describing the external morphology of one mineral form, while polymorphs represent entirely different structural forms of the same chemical substance.
Factors Influencing Crystal Habit Formation
Crystal habit formation is influenced by factors such as temperature, solvent type, concentration, and impurities, which determine the external shape of a crystal. Polymorphs exhibit different crystal habits due to variations in molecular packing and intermolecular forces, leading to distinct physical properties. Control over crystallization conditions allows for selective growth of specific polymorphs with desirable crystal habits for pharmaceutical and material science applications.
Causes and Types of Polymorphism
Crystal habit refers to the external shape or morphology that crystals grow into, influenced by growth conditions such as temperature, pressure, and solution chemistry. Polymorphism occurs when a substance can crystallize into more than one crystal structure or form, driven by variations in thermodynamic parameters like temperature, pressure, or the presence of solvents or impurities. Types of polymorphism include enantiotropy, where forms reversibly transform into each other, and monomorphism, where one form is stable and the other metastable depending on external conditions.
Real-World Examples of Crystal Habits
Crystal habits describe the external shapes crystals form during growth, influenced by environmental conditions, while polymorphs are distinct structural forms of the same chemical compound. Quartz commonly exhibits a hexagonal prismatic crystal habit, whereas diamond, a polymorph of carbon, shows an octahedral habit due to its unique atomic arrangement. Real-world examples include gypsum crystals forming tabular habits and calcite displaying rhombohedral habits, illustrating how crystal habits reflect underlying molecular structure and growth conditions.
Common Polymorphic Minerals and Their Structures
Common polymorphic minerals such as quartz, calcite, and carbon exhibit distinct crystal habits due to their varied atomic arrangements despite sharing identical chemical compositions. Quartz commonly appears in hexagonal prisms, whereas its polymorph tridymite forms more tabular or flaky crystals, reflecting differences in silica tetrahedral linkage. Calcite and aragonite both consist of calcium carbonate but display rhombohedral and orthorhombic crystal systems respectively, directly influencing their unique external crystal habits in natural environments.
Importance in Mineral Identification and Classification
Crystal habit, referring to the external shape and form of a mineral's crystals, provides key visual clues essential for mineral identification and classification alongside polymorphs, which are minerals with identical chemical compositions but different internal crystal structures. Recognizing distinct crystal habits helps differentiate minerals with similar appearances, while understanding polymorphism reveals variations in physical properties like hardness and stability, critical for accurate mineral characterization. Both concepts are fundamental in petrology and mineralogy, facilitating the precise categorization of minerals in geological studies and resource exploration.
Applications and Implications in Geology and Material Science
Crystal habit refers to the characteristic external shape of a mineral crystal, influencing identification and classification in geology, while polymorphs are minerals with the same chemical composition but different crystal structures, significantly affecting material properties. Understanding crystal habit aids geologists in interpreting formation environments, whereas polymorph analysis is crucial in material science for tailoring physical properties like hardness, stability, and conductivity. The application of polymorph control enables the development of advanced materials with optimized performance, while crystal habit provides insights into geological processes and mineral genesis.
Crystal Habit Infographic
 libterm.com
libterm.com