Geometric isomers are molecules with the same molecular formula but different spatial arrangements of atoms due to restricted rotation around a double bond or ring structure. These isomers often exhibit distinct physical and chemical properties, influencing reactivity and biological activity. Explore the rest of this article to understand how geometric isomers impact chemical behavior and practical applications.
Table of Comparison
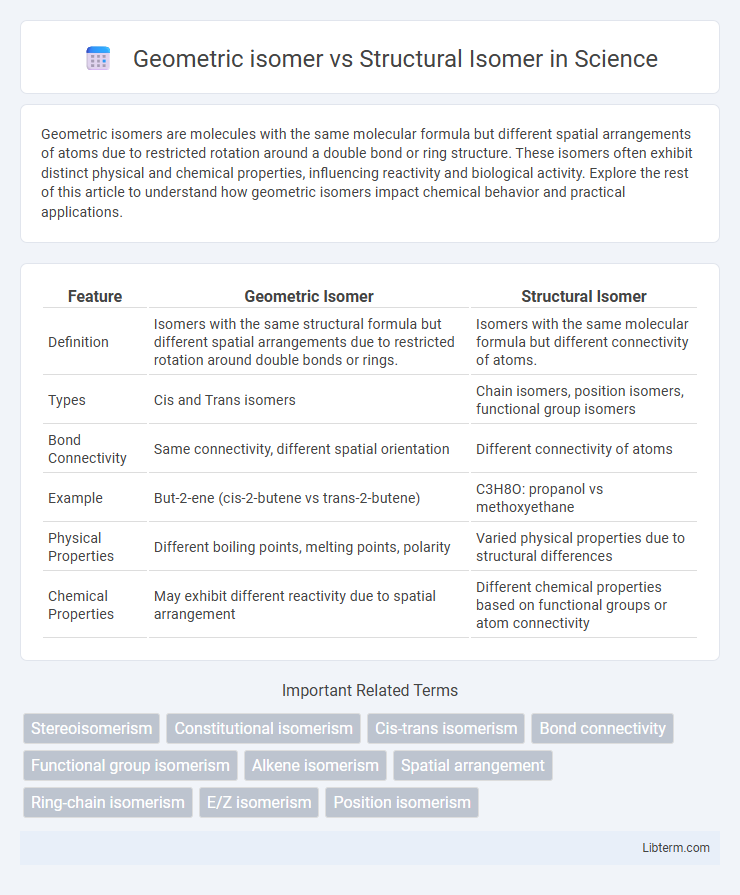
| Feature | Geometric Isomer | Structural Isomer |
|---|---|---|
| Definition | Isomers with the same structural formula but different spatial arrangements due to restricted rotation around double bonds or rings. | Isomers with the same molecular formula but different connectivity of atoms. |
| Types | Cis and Trans isomers | Chain isomers, position isomers, functional group isomers |
| Bond Connectivity | Same connectivity, different spatial orientation | Different connectivity of atoms |
| Example | But-2-ene (cis-2-butene vs trans-2-butene) | C3H8O: propanol vs methoxyethane |
| Physical Properties | Different boiling points, melting points, polarity | Varied physical properties due to structural differences |
| Chemical Properties | May exhibit different reactivity due to spatial arrangement | Different chemical properties based on functional groups or atom connectivity |
Introduction to Isomerism
Isomers are compounds with the same molecular formula but different arrangements of atoms, leading to distinct properties. Geometric isomers, a type of stereoisomer, differ in spatial arrangement around a double bond or ring structure, affecting physical and chemical behavior without changing connectivity. Structural isomers, also known as constitutional isomers, vary in the actual connectivity of atoms, resulting in different functional groups or carbon skeletons.
Defining Geometric Isomers
Geometric isomers are a type of stereoisomer where compounds have the same molecular formula and connectivity but differ in the spatial arrangement of atoms or groups around a rigid structure, typically a double bond or a ring. These isomers arise due to restricted rotation, producing distinct cis (same side) and trans (opposite side) configurations. Unlike structural isomers, which differ in the order of atomic connectivity, geometric isomers maintain the same connections but vary in three-dimensional orientation, impacting physical and chemical properties.
Understanding Structural Isomers
Structural isomers are compounds with the same molecular formula but different connectivity of atoms, resulting in distinct chemical and physical properties. Unlike geometric isomers, which differ only in spatial arrangement around a double bond or ring, structural isomers vary in the sequence of bonded atoms, including chain isomers, position isomers, and functional group isomers. Understanding structural isomers is crucial for identifying how variations in atomic connectivity influence reactivity and functionality in organic chemistry.
Key Differences Between Geometric and Structural Isomers
Geometric isomers differ in the spatial arrangement of atoms around a double bond or ring structure, leading to variations such as cis and trans forms, whereas structural isomers vary in the connectivity of atoms, resulting in different chemical structures altogether. Geometric isomers have the same molecular formula and bonding sequence but differ in their 3D orientation, while structural isomers differ in both molecular formula arrangement and bonding patterns. These key differences influence their physical properties, reactivity, and biological activity significantly.
Examples of Geometric Isomerism
Geometric isomers, also known as cis-trans isomers, occur due to restricted rotation around a double bond or within a ring structure, with common examples including cis-2-butene and trans-2-butene, where the positions of methyl groups differ relative to the double bond. Structural isomers, in contrast, differ in the connectivity of atoms, such as butanol and isobutanol, which have the same molecular formula (C4H10O) but distinct structural arrangements. Geometric isomerism significantly affects physical and chemical properties, illustrated by the different boiling points and reactivities of cis and trans isomers in alkenes and cyclic compounds.
Common Types of Structural Isomerism
Geometric isomers differ in the spatial arrangement of atoms around a double bond or ring, while structural isomers have different connectivity of atoms. Common types of structural isomerism include chain isomerism, where carbon skeletons vary; positional isomerism, involving different positions of functional groups; and functional group isomerism, where compounds possess distinct functional groups. Understanding these isomer types is crucial for predicting physical and chemical properties in organic chemistry.
Methods for Identifying Isomer Types
Geometric isomers are identified primarily through techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy, which reveals differences in the spatial arrangement of atoms around a double bond or ring structure. Structural isomers can be distinguished using mass spectrometry and Infrared (IR) spectroscopy, which provide insights into different bonding patterns and functional groups. Chromatography methods, including Gas Chromatography (GC) and High-Performance Liquid Chromatography (HPLC), further separate isomers based on polarity and molecular interactions, aiding in accurate identification.
Chemical Properties: Geometric vs Structural Isomers
Geometric isomers exhibit different chemical properties due to variations in spatial arrangement, influencing reactivity in processes like addition reactions and interactions with catalysts. Structural isomers show distinct chemical behaviors arising from differences in atom connectivity, resulting in variations in functional groups and bonding patterns. These differences lead to divergent boiling points, solubilities, and reaction mechanisms between geometric and structural isomers.
Importance of Isomerism in Chemistry
Isomerism, including geometric isomers and structural isomers, plays a crucial role in chemistry by influencing molecular properties and reactivity. Geometric isomers differ in spatial arrangement around a double bond or ring, impacting physical and chemical characteristics, while structural isomers vary in the connectivity of atoms, resulting in distinct compounds with unique functions. Understanding these isomers is essential for drug design, material science, and biochemical processes where molecular shape and connectivity determine effectiveness and interactions.
Real-World Applications of Geometric and Structural Isomers
Geometric isomers play a crucial role in the pharmaceutical industry, as their different spatial arrangements can drastically affect drug efficacy and receptor binding, exemplified by cis-platin in cancer treatment. Structural isomers are fundamental in materials science, where variations in molecular connectivity influence the properties of polymers like polyethylene and polystyrene, impacting product durability and flexibility. Both isomer types are essential in agrochemicals, where geometric isomers determine pesticide activity, while structural isomers offer diverse chemical properties for herbicide formulations.
Geometric isomer Infographic
 libterm.com
libterm.com